Abstract
Although rabbits are common domestic pets, severe respiratory allergic reactions to rabbits in households are unusual. Ory c 1, a 17-kDa glycoprotein found in saliva and fur, has previously been identified as a major rabbit allergen. In this report, we describe the cases of three patients with rabbit allergy who presented with asthma and/or rhinitis while living in households with detectable levels of serum-specific IgE and major IgE binding components. Three patients with rabbit allergy and 18 unexposed nonatopic healthy controls were enrolled. Enzyme-linked immunosorbent assays (ELISA) for serum-specific IgE and IgG4 to rabbit epithelium and inhibition ELISA were performed followed by sodium dodecye sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and IgE immunoblotting. All three patients with rabbit allergy had high serum-specific IgE antibody levels compared with controls. The results of the inhibition ELISA showed significant inhibition with the addition of rabbit epithelium, whereas no significant inhibition was noted with the addition of cat and dog epithelia. Two IgE-binding components with molecular weights of 16 kDa and 67.5 kDa were identified by IgE immunoblotting. In conclusion, rabbit exposure may induce IgE-mediated bronchial asthma and/or rhinitis in domestic settings.
The development of allergic rhinitis, conjunctivitis, and asthma with rabbit exposure is common among scientists, technicians, and laboratory animal handlers (1). There was a report of a case of occupational allergic rhinitis working for the woolen fabric factory with rabbit fur in Korea (2). In a large epidemiologic study in Japan, allergic reactions were reported in 30% of laboratory animal workers with exposure to rabbits (3). However, few studies have investigated the specific allergens involved in sensitization to rabbits. Ory c 1, a 17-kDa glycoprotein found in saliva and fur, has previously been identified as a major rabbit allergen (4, 5). Rabbit serum albumin and Ag2 have also been identified as allergens (5). Although rabbits are common domestic pets, the development of allergic asthma and/or rhinitis attributable to rabbits at home is unusual, and few studies have evaluated the allergenic relationship between rabbits and other pets such as cats and dogs.
Here, we report the identification of serum-specific IgE and IgG4 antibodies to rabbit epithelium and IgE binding components in three cases of allergic asthma and/or rhinitis resulting from rabbit exposure at home. Furthermore, to elucidate the allergenic cross-reactivity between rabbits and other furry animals, we performed inhibition enzyme-linked immunosorbent assays (ELISA).
A 20-yr-old woman presented with a history of severe breathing difficulty, coughing, and wheezing for several days. She had lived in a school dormitory during the past year without incident but experienced breathing difficulty and coughing whenever she visited her family's house. Her family had kept two rabbits as pets during the past 3 yr. On admission, the patient's forced expiratory volume in one second (FEV1) level was 50%, and the sputum eosinophil level was 80%. Her FEV1 level improved to 100% after 3 days of treatment with systemic steroids and bronchodilators. These results suggested bronchial asthma, so methacholine challenge test was not needed to confirm asthma in this patient. Allergy skin prick tests with 55 common inhalant and food allergens (rabbit epithelium not included) showed negative results. The patient had no history or family history of allergic diseases. To evaluate the presence of rabbit-induced bronchial asthma, peak expiratory flow rate (PEFR) and serum-specific IgE to rabbit epithelium were measured. The results of PEFR monitoring showed significant decreases in PEFR values at home compared with those at school (Fig. 1), even she did not take asthma medication at school. Serum-specific IgE to rabbit epithelium was detected (31.9 kU/L; CAP system, Pharmacia, Uppsala, Sweden). After removal of the rabbits from her family's home, the patient's respiratory symptoms improved without the need for medication.
A 37-yr-old woman presented with allergic conjunctivitis and eczema, and a history of rabbit allergy 8 yr before. After breeding rabbits at her home during the past 6 months, she developed shortness of breath, wheezing, cough, rhinorrhea, and sneezing. After removal of the rabbits from her home, her asthma and rhinitis improved. A methacholine challenge test showed a positive reaction, with a PC20 value of 0.094 mg/mL. Allergy skin prick tests with 55 common inhalant and food allergens showed positive reactions, with an A/H ratio of 4+ in Dermatophagoides pteronyssinus, 4+ in D. farine, 4+ in dog, and 2+ in cat. Serum-specific IgE was detected for rabbit, dog, and cat epithelia (41.0, 4.15, and 0.83 kU/L, respectively). Her family history was significant for parents and a sister who had allergic rhinitis. She also bred dogs and birds but denied experiencing any symptoms on exposure to these animals.
A 33-yr-old man with allergic rhinitis presented with a 2-month history of coughing, intermittent shortness of breath, and wheezing. He had kept a rabbit as a pet during the past 4 yr. After living with the rabbit for 2 yr, his rhinitis symptoms became aggravated whenever he stayed at home or handled the animal. He denied experiencing any asthma symptoms on exposure to the rabbit. His family history was significant for a grandfather with asthma. A methacholine challenge test showed a positive reaction, with a PC20 value of 1.25 mg/mL. Allergy skin prick tests with 55 common inhalant and food allergens showed positive reactions, with an A/H ratio of 3+ in D. pteronyssinus, 2+ in D. farine, 4+ in mugwort, and 5+ in ragweed. The serum-specific IgE to rabbit epithelium was 4.86 kU/L.
The three cases are summarized in Table 1.
We measured the levels of serum-specific IgE (or IgG4) to rabbit epithelium in three patients with rabbit allergy and in 18 healthy non-atopic controls. Microtiter plates (Costar, Corning, NY, U.S.A.) were Hamburg coated with 100 µL of rabbit epithelium (Allergopharma, Hamburg, Germany) and stored at 4℃ overnight. Plates were washed 3 times between steps with 0.05% phosphate-buffered saline-Tween (PBS-T). Blocking was performed by using 10% fetal bovine serum albumin for 1 hr at room temperature. The patients' sera (undiluted, 50 µL/well) were added and incubated for 1 hr at room temperature. After washing, 100 µL of 1:1,000 biotin-labeled goat anti-human IgE (or IgG4) antibodies (Sigma, St. Louis, MO) were added and incubated for 1 hr at room temperature. IgE reactivity was detected by colorimetric reaction using 3,3', 5,5'-tetramethyl benzidine and H2O2 as substrate. The absorbance at 450 nm was read using an automated microplate reader. A competitive inhibition ELISA was performed to determine the specificity of the IgE binding to rabbit epithelium and to identify allergenic cross-reactivity with cat and dog epithelia. A sera pool from two patients with high-specific IgE levels to rabbit epithelium was preincubated with 0, 1, 5, and 10 µg/mL of rabbit, cat, dog epithelia (Allergopharma), and D. pteronyssinus. The inhibition percentage of the specific IgE binding was expressed as 100-[(absorbance of samples reincubated with allergens/ absorbance of samples pre-incubated with PBS)×100%].
Rabbit epithelium (0.75 µg/well) was boiled in sample buffer (0.5 M Tris [pH 6.8], glycerol, 10% SDS, 0.5% bromophenol blue, 2.5% β-mercaptoethanol) for 5 min. Standard markers (4 to 250 kDa, Invitrogen, San Diego, CA, U.S.A.) and rabbit epithelia were loaded onto a 10% Trisglycine gel for antigen separation. Electrophoresis was performed with the Mini Protean II cell (Bio-Rad Laboratories, Hercules, CA, U.S.A.) for 120 min at 125 V. The gel was fixed and stained with Coomasie Brilliant blue. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)-separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore Co., Bedford, MA, U.S.A.) at 200 mA for 2 hr. Nonspecific binding sites on the nitrocellulose membrane were blocked by incubation with 5% skim milk in Tris-buffered saline with Tween 20 (TBST) for 2 hr, and the membranes were incubated with sera from patients and controls for 2 hr at room temperature. After washing 4 times with TBST, the membranes were incubated with alkaline phosphatase-labeled conjugated goat anti-human IgE (or IgG4) antibody (Sigma) diluted 1:1,000 with 5% skim milk in TBST for 1 hr. After washing, the colorimetric reaction was developed with the BCIP/NBT alkaline phosphatase substrate (Sigma).
When the mean+3 SD was defined as the cut-off value for the controls, all three patients with rabbit allergy showed positive results for specific IgE antibody, and two of the patients showed positive results for specific IgG4 antibody (Fig. 2). In addition, the specific IgE levels were higher in the two patients with asthma than in the patient with rhinitis alone.
Inhibition ELISA using the sera pool from the patients with rabbit allergy showed marked dose-dependent inhibition with the addition of rabbit antigen, whereas minimal inhibition was noted with the addition of cat, dog, and D. pteronyssinus antigens (Fig. 3).
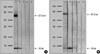
Rabbit epithelium was resolved into two bands, 16 kDa and 67.5 kDa, by SDS-PAGE and IgE immunoblot analysis; a 16-kDa protein band was noted in the sera of all three patients, and a 67.5-kDa band was noted in the sera of one patient (Fig. 4A). The IgG4 immunoblot analysis showed a similar pattern (Fig. 4B).
IgE-mediated allergic sensitization is commonly experienced by individuals in regular contact with rabbits in laboratory and domestic settings (1, 2). Although rabbits are common domestic pets, there have been few reports of severe respiratory allergy resulting from household exposure to rabbits. One report described an atopic child who had developed anaphylaxis following inhalant exposure to a rabbit at home (6), and another report described an 18-yr-old woman who had developed severe respiratory allergy by indirect exposure to rabbit epithelia carried on the clothes of a colleague who kept a rabbit as a pet (7). We described the cases of three patients with rabbit allergy who presented with asthma and/or rhinitis. Although allergy skin test with rabbit epithelium was not available in our hospital, specific IgE antibodies to rabbit epithelium were detected in the sera of the patients by both ELISA and CAP. Furthermore, high serum-specific IgG4 antibody levels were detected in two patients, which might have resulted from a parallel immune response to rabbit allergen. Risk factors for the development of laboratory animal allergies include atopy, familial history of allergic diseases, and environmental susceptibility (1). However, one case in the present study showed pure rabbit-induced asthma in a patient without a family history of allergic diseases, atopy, or underlying allergic diseases, indicating that even a non-atopic person without a family history of allergic diseases may develop severe animal-induced asthma from household exposure. Also, as rabbits are frequently kept as domestic pets theses days, rabbit epithelium should be considered one of common inhalant animal allergens.
To date, several rabbit (Oryctolagus cuniculus) allergens have been identified in saliva, fur, urine, dander, and dust (4, 5, 8, 9). Ohman et al. reported allergens with molecular weights ranging from 18 to 38 kDa from rabbit pelt extracts (8). Longbottom et al. identified several allergens, including a 17-kDa glycoprotein, Ag R1 (referred to as Ory c 1), Ag2, serum albumin, and several other bands, using crossed immunoelectrophoresis (4, 5). Recently, Baker et al. showed that 18-kDa and 21-kDa allergens (previously identified as Ory c 1 and Ag2, respectively) belong to the lipocalin superfamily and have significant sequence similarity to odorant binding proteins (9). Lipocalins play a role in the binding and transport of small hydrophobic molecules such as pheromones (10). In this report, IgE immunoblot analysis showed two distinct proteins, 16 kDa and 67.5 kDa, which might have been Ag R1 and rabbit serum albumin, respectively. The 16-kDa band reacted with serum IgE and IgG4 in all three patients, whereas the 67.5-kDa band reacted only in one patient. Consistent with previous reports, Ag R1 was identified as a major rabbit allergen, and rabbit serum albumin did not appear to be a major allergen (4, 5, 9). This latter finding is in contrast to reports showing that serum albumin is an important allergen in cat, dog, and horse sera (1, 10).
It is well recognized that albumins from different species may cause allergenic cross-reactions (11, 12). In this report, the possibility of cross-reactivity between rabbit allergen and cat and dog allergens was shown to be very low. Although we used commercially available animal epithelia instead of crude extracts, we were able to find the 68-kDa glycoprotein band, which might have been serum albumin, in cat and dog epithelia by SDS-PAGE (data not shown). One explanation for this observation is the presence of specific IgE epitopes for certain albumins. Furthermore, a significant variability has been shown in IgE reactivities to variant albumins, despite a high degree of sequence similarity among the different albumins and overall cross-reactivity of IgE reactivities in patients with allergies (11). Additional studies are required to elucidate the allergenic cross-reactivity among albumins and its clinical significance.
In conclusion, we described the cases of three patients who experienced rabbit-induced bronchial asthma and/or rhinitis in the home environment. Serum-specific IgE and IgG4 antibodies to rabbit allergens were demonstrated, and a 17-kDa glycoprotein was confirmed as a major rabbit allergen. As rabbits have become increasingly popular domestic pets, rabbit allergies experienced at home or at the workplace have become more common. Physicians should be aware that rabbit exposure may cause severe respiratory allergic reactions even in non-atopic individuals.
Figures and Tables
Fig. 1
PEFR values markedly decreased while at home with the rabbit. PEFR, peak expiratory flow rate.

Fig. 2
Specific IgE (A) and IgG4 (B) binding to rabbit epithelium according to ELISA using sera from three patients with rabbit allergy (●) and unexposed healthy controls (○). Solid lines represent mean + 3 SD, and dotted lines represent mean values of the healthy controls.

Fig. 3
Inhibition ELISA using a sera pool from three patients with rabbit allergy with the addition of rabbit (●), cat (■), and dog (▲) epithelia and D. pteronyssinus (◆) extracts.

References
1. Bush RK, Wood RA, Eggleston PA. Laboratory animal allergy. J Allergy Clin Immunol. 1998. 102:99–112.

2. Lee JC, Lee KS, Park EC, Kang MH, Kang SY. J Asthma Allergy Clin Immunol. 1990. 10:71–76.
3. Aoyama K, Ueda A, Manda F, Matsushita T, Ueda T, Yamauchi C. Allergy to laboratory animals: an epidemiologic study. Br J Ind Med. 1992. 49:41–47.
4. Price JA, Longbottom JL. Allergy to rabbits. II. Identification and characterization of a major rabbit allergen. Allergy. 1988. 43:39–48.
5. Warner JA, Longbottom JL. Allergy to rabbits. III. Further identification and characterization of rabbit allergens. Allergy. 1991. 46:481–491.
6. Prince E, Zacharisen MC, Kurup VP. Anaphylaxis to rabbit: a case report. Ann Allergy Asthma Immunol. 1998. 81:272–273.

7. Liccardi G, D'Amato G, Canonica GW, Dente B, Passalacqua G. Severe respiratory allergy induced by indirect exposure to rabbit dander: a case report. Allergy. 2004. 59:1237–1238.

8. Ohman JL Jr, Lowell FC, Bloch KJ. Allergens of mammalian origin. II. Characterization of allergens extracted from rat, mouse, guinea pig, and rabbit pelts. J Allergy Clin Immunol. 1975. 55:16–24.
9. Baker J, Berry A, Boscato LM, Gordon S, Walsh BJ, Stuart MC. Identification of some rabbit allergens as lipocalins. Clin Exp Allergy. 2001. 31:303–312.

10. Stewart GA, Robinson C. Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Allergen structure and function. Middleton's allergy. Principles and practices. 2003. 6th ed. Philadelphia: Mosby Inc.;585–609.
11. Spitzauer S, Pandjaitan B, Söregi G, Mühl S, Ebner C, Kraft D, Valenta R, Rumpold H. IgE cross-reactivities against albumins in patients allergic to animals. J Allergy Clin Immunol. 1995. 96:951–959.

12. Hilger C, Kohnen M, Grigioni F, Lehners C, Hentges F. Allergic cross-reactions between cat and pig serum albumin. Study at the protein and DNA levels. Allergy. 1997. 52:179–187.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download