Abstract
Extramedullary plasmacytoma of the liver is a very rare tumor. Although a few cases of extramedullary plasmacytoma of the liver have been reported, we could not find any report on truly localized extramedullary plasmacytoma of the liver in the literature. The patient was a 63-yr-old man who exhibited a solitary liver mass on dynamic computed tomography and magnetic resonance imaging. Histologically, the tumor was composed of mature plasma cells with mild atypia. Immunohistochemistry demonstrated monoclonal IgG and Kappa light chain expression. Bone marrow examination revealed no abnormalities. There was no evidence of a monoclonal protein in the serum and urine, lytic bone lesions, anemia, renal insufficiency, and hypercalcemia. The patient was treated with 5,000 cGy of radiotherapy, and the tumor disappeared 6 months after treatment.
Solitary extramedually plasmacytomas represent approximately 3% of all plasma cell neoplasms (1). They most commonly affect men in their elderly 60s and occur in the upper respiratory tract (paranasal sinuses, nose, nasopharynx, and tonsils). They also occur in lymph nodes, lung, thyroid, gastrointestinal tract, liver, spleen, pancreas, testes, breast, or skin (2). Although extramedually plasmacytomas are not common in newly diagnosed multiple myeloma, classic myeloma must be excluded by thorough staging. A monoclonal protein is detected in the serum and urine in approximately 25% of patients (3-8). A monoclonal protein in the serum and urine, lytic bone lesions, anemia, renal insufficiency, and hypercalcemia should be excluded. It is most unusual for this condition to present in the liver as a primary solitary lesion. Only a few cases of primary hepatic extramedually plasmcytoma have been reported; however, these cases revealed monoclonal gammopathy in the serum and/or urine (9-12). Here we present the first case of truly localized solitary hepatic extramedually plasmacytoma without evidence of myeloma elsewhere.
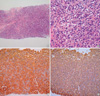
A 63-yr-old man was referred from a primary clinic due to a hepatic mass on ultrasonography. He had well-controlled hypertension and diabetes. Physical examination was unremarkable. Computed tomography (CT) scans (Sensation 4, Siemens Medical System, Forchheim, Germany) were obtained after injection of 150 mL of ionic contrast material (Iopamiro 300; Bracco, Milano, Italy). Contrast-enhanced CT of the liver was performed with 2.5 mm detector collimation, 5 mm slice thickness, 15 mm table feed, 6 pitch, 1.5 mm reconstruction during the arterial phase (10 sec after 100 HU aortic enhancement) and portal venous phase (72 sec after contrast injection). The arterial phase images showed a 2×2 cm-sized well enhanced mass in segment VI of the liver. This mass showed hypodensity on the portal venous phase (Fig. 1A, B). Liver magnetic resonance imaging (MRI) revealed a well-defined mass in Segment VI. This mass showed low signal intensity on T1-weighted image and high signal intensity on T2-weighted image (Fig. 1C, D). Initial impression of the contrast-enhanced CT suggested the possibility of hypervascular hepatic tumors including adenoma, focal nodular hyperplasia, and hepatocellular carcinoma. Hematological indexes, liver function test, and renal functions were within the normal limits. The patient was negative for viral markers, and α-fetoprotein was not increased. A needle biopsy of the liver mass and bone marrow trephine biopsies were performed. The histological examination of the liver biopsy disclosed diffuse solid proliferation of monotonous small round to ovoid cells without any lymphoid cells, eosinophils, or fibroblasts. They had eccentric cytoplasm and round nuclei with peripheral condensation of chromatin. Occasional binucleated forms were noted. The diagnosis after immunohistochemical staining was plasmacytoma with IgG and kappa type (Fig. 2). The bone marrow biopsy specimens showed 30% marrow cellularity but were otherwise normal. Serum calcium, β-2 microglobulin, and serum and urine protein immunoelectrophoresis were normal. Skeletal radiography revealed no abnormalities. The patient was treated with 5,000 cGy of radiotherapy. Follow-up contrast-enhanced CT after six month showed treatment response of the hepatic mass (Fig. 3).
Most patients with plasma cell neoplasia have generalized disease at diagnosis, i.e. multiple myeloma (3). Solitary extramedullary plasmacytoma represents approximately 3% of all plasma cell neoplasm (4, 5). Although solitary extramedullary plasmacytoma can arise throughout the body, almost 90% arise in the head and neck, especially in the upper respiratory tract including the nasal cavity, sinuses, oropharynx, salivary glands, and larynx (2, 6, 13-15). The second most frequent site is the gastro intestinal tract. A variety of other sites can rarely be involved, including testis, bladder, urethra, breast, ovary, pleura, thyroid, orbit, brain, and skin (1).
The diagnosis of solitary extramedullary plasmacytoma requires the demonstration of a monoclonal plasma cell infiltrate without any evidence of multiple myeloma elsewhere (5). The patient described here is a man who presented with a solitary hepatic extramedually plasmacytoma without evidence of myeloma elsewhere including no monoclonal protein in the serum or urine. Only a few cases of primary hepatic extramedually plasamcytoma have been reported; however, these cases revealed monoclonal gammopathy in the serum and/or urine (9-12). Brinch et al. (6) reported only 3 out of 18 patients with extramedullary plasmacytoma had a systemic M-protein; however, the presenting sites of extramedullary plasmacytomas included in the report were the upper respiratory tract (epipharynx, nose, paranasal sinuses, and thorax). This is the first report of truly localized solitary hepatic extramedually plasmacytoma without evidence of monoclonal gammopathy in the serum and urine. The reason for the diverse incidence of systemic monoclonal gammopathy between the presenting sites is not documented but could be related with the time at the diagnosis of the plasmacytomas. The predictive value of the presence or absence of an M-component for the later development of myelomatosis was poor (6).
The current treatment of choice is radiotherapy because these tumors are highly radiosensitive. A radiation dose of 4,000 to 5,000 cGy over 4 to 5 weeks was associated with a less than 5% risk of local recurrence (7). No local recurrences were observed with radiation doses of at least 4,500 cGy (5). In our patient, the plasmacytoma was located on the tip of the right lobe, and the patient was treated with 5,000 cGy of radiotherapy encompassing the primary tumor with an ample margin. For extramedually plasmacytomas at sites other than head and neck, complete surgical removal should be considered if feasible (3). The diagnosis of patients with extramedually plasmacytomas is usually made after surgical excision (9, 10). It is recommended that patients with positive surgical margins should receive adjuvant radiotherapy, but adjuvant radiotherapy is not recommended for patients who have undergone complete surgical excision with a negative margin (3). There is no evidence that adjuvant chemotherapy improves patients' outcome (5). As a result, extramedullary plasmacytoma has a relatively favorable prognosis, and a solitary extramedullaty plasmacytoma can be a curable disease by meticulous staging. At least 70% of these patients remain disease-free at 10 yr, and fewer than 30% develop a distant failure in the form of multiple myeloma or multiple extramedulary tumors (2, 4-8).
Figures and Tables
Fig. 1
On contrast-enhanced computed tomography, the arterial phase image (A) shows a 2×2 cm-sized well enhanced mass in segment VI of the liver. This mass shows hypodensity on the portal venous phase (B). On magnetic resomance imaging, the T1-weighted image (C) shows a low signal intensity mass in segment VI. This mass shows high signal intensity in a T2-weighted image (D).
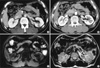
Fig. 2
The liver shows diffuse solid proliferation of monotonous small round to ovoid cells (A: H&E, ×100). They have eccentric cytoplasm and round nuclei with peripheral condensation of chromatin (B: H&E, ×400). Immunohistochemical stains of the hepatic mass exhibit monoclonality for IgG heavy chain (C: ABC, ×200) and kappa light chain (D: ABC, ×200).
References
1. Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullaty plasmacytoma. Hematol Oncol Clin North Am. 1999. 13:1249–1257.
2. Galieni P, Cavo M, Pulsoni A, Avvisati G, Bigazzi C, Neri S, Caliceti U, Benni M, Ronconi S, Lauria F. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000. 85:47–51.
3. Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Samson D. Guidelines Working Group of the UK Myeloma Forum. British Committee for Standards in Haematology. British Society for Haematology. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol. 2004. 124:717–726.

4. Knowling MA, Harwood AR, Bergsagel DE. Comparision of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol. 1983. 1:255–262.
5. Shih LY, Dunn P, Leung WM, Chen WJ, Wang PN. Localised plasmacytomas in Taiwan: comparison between extramedullary plasmacytoma and solitary plasmacytoma of bone. Br J Cancer. 1995. 71:128–133.

6. Brinch L, Hannisdal E, Foss-Abrahamsen A, Kvaloy S, Langholm R. Extramedullary plasmacytomas and solitary plasma cell tumours of bone. Eur J Haematol. 1990. 44:132–135.

7. Mayr NA, Wen BC, Hussey DH, Burns CP, Slaples JJ, Doornbos JF, Vigliotti AP. The role of radiation therapy in the treatment of solitary plasmacytomas. Radiother Oncol. 1990. 17:293–303.

8. Holland J, Trenknor DA, Wasserman TH, Fineberg B. Plasmacytoma: Treatment results and conversion to myeloma. Cancer. 1992. 69:1513–1517.

9. Dohy H, Abe T, Takata N, Fujimura K, Taketomi Y, Kuramoto A, Harada T, Hattori T, Enzan H. Successful hepatectomy for solitary plasmacytoma. N Engl J Med. 1979. 300:1218–1219.

10. Weichhold W, Labouyrie E, Merlio JP, Masson B, Mascarel A. Primary extramedually plasmacytoma of the liver. A case report. Am J Surg Pathol. 1995. 19:1197–1202.
11. Petrucci MT, Tirindelli MC, De Muro M, Martini V, Levi A, Mandelli F. Extramedually liver plasmacytoma a rare presentation. Leuk Lymphoma. 2003. 44:1075–1076.
12. Hyun DW, Park SW, Baik JH, Kim DH, Jung JT, Shin DG, Sohn SK, Lee KB. A case of multiple myeloma with multiple intrahepatic extramedually plasmacytomas. Korean J Hematol. 1999. 34:143–147.
13. Wax MK, Yun KN, Omar RA. Extramedullary plasmacytomas of the head and neck. Otolaryngol Head Neck Surg. 1993. 109:877–885.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download