Abstract
Because high levels of cortisol are frequently observed in patients with septic shock, low levels of serum cortisol are considered indicative of relative adrenal insufficiency (RAI). This study was performed to investigate whether pretest clinical characteristics, including basal serum cortisol levels, are predictive of serum cortisol response to corticotropin and whether basal cortisol levels have a prognostic significance in patients with septic shock. We performed a retrospective analysis of 68 patients with septic shock who underwent short corticotropin stimulation testing. RAI was defined as an increase in cortisol level <9 µg/dL from baseline, and results showed that 48 patients (70.6%) had this insufficiency. According to the univariate analysis, the RAI group had significantly higher simplified acute physiology score II (SAPS II) and sequential organ failure assessment (SOFA) scores than the non-RAI group. The incidence of RAI was the same regardless of the basal serum cortisol level (p=0.447). The hospital mortality rate was 58.8% and was not significantly different between the RAI and non-RAI groups. However, a high basal serum cortisol level (≥30 µg/dL) was significantly associated with in-hospital mortality. In conclusion, our data suggest that basal serum cortisol levels are not predictive of serum cortisol response to corticotropin but have a significant prognostic value in patients with septic shock.
Septic shock is accompanied by activation of the hypothalamic-pituitary-adrenal axis, as demonstrated by an increased serum cortisol concentration (1), and this activation is the essential component of the general adaptation to stress. Cortisol has a vital supportive role in the maintenance of vascular tone, endothelial integrity, vascular permeability, and the distribution of total body water within the vascular compartment (2-4), and also potentiates the vasoconstrictor actions of catecholamines (2, 4). Cortisol levels considered normal or above normal may not be sufficient in critically ill patients (5), and insufficient cortisol secretion related to illness severity is referred to as relative adrenal insufficiency (RAI).
Increasing evidence indicates that RAI occurs in critically ill patients with septic shock and that low-dose corticosteroids may improve outcomes in these patients (6). Diagnosis of RAI is difficult because the expected cortisol levels vary with the type and severity of disease (5, 7) and with different serum levels of binding proteins (8). Cortisol demonstrates different degrees of tissue resistance (9) and cortisol levels vary with the time of blood sampling because of the pulsatile nature of cortisol secretion. In addition, the incidence of RAI may vary according to the diagnostic test performed and the criteria used to establish the diagnosis. In several studies, low levels of random serum cortisol were used to diagnose RAI, using a cutoff value between 15 and 25 µg/dL (10-13). However, increases in cortisol levels <9 µg/dL after corticotropin stimulation have been associated with vascular unresponsiveness to catecholamines (14, 15) and with an increased risk of death (5). Cortisol replacement has been shown to restore vessel reactivity to vasopressor agents and to improve survival (6, 14). Thus, although increases in the cortisol level <9 µg/dL after corticotropin stimulation is most often used to define RAI, rapid ACTH stimulation test takes time and needs three samples of blood making it cumbersome to perform. If cortisol response to corticotropin stimulation can be predicted by some clinical factors, for example, basal cortisol level, it will be very helpful for clinicians at the bedside to make a decision, but up to now no such variables have been defined.
The aim of this study was to determine whether pretest clinical factors, including basal cortisol levels, are predictive of cortisol response to standard short corticotropin testing and to assess the significance of basal cortisol levels as a prognostic indicator in patients with septic shock.
Sixty-eight patients with septic shock who underwent short corticotropin stimulation testing at Samsung Medical Center, in Seoul, Republic of Korea, between January 2004 and August 2005 were retrospectively enrolled in the study. All of the patients met the Society of Critical Care Medicine/American College of Chest Physicians criteria of septic shock (16). None of the patients were taking corticosteroids, etomidate, ketoconazole, or other drugs known to suppress adrenal function.
The short corticotropin stimulation test was performed by administering 250 µg of synthetic corticotropin intravenously and obtaining serum samples for cortisol before and 30 and 60 min following corticotropin administration. RAI was diagnosed when the peak cortisol concentration after corticotropin administration was <9 µg/dL from baseline either at 30 min or 60 min.
The patient's clinical and laboratory data were recorded with a retrospective chart review. The severity of illness was assessed by simplified acute physiology score II (SAPS II) and sequential organ failure assessment (SOFA) score at the time of short corticotropin stimulation test. Statistical analyses were performed with SPSS 11.0 (SPSS, Chicago, IL, U.S.A.). Values were expressed as means±standard deviations, or as numbers (percentages) in the text and tables. Chi-square analysis with Fisher's exact test (when appropriate) was used to compare categorical data. Continuous data were compared with Student's t-test. Logistic regression was used to calculate the odds ratios of risk factors and a log-rank test was used to evaluate factors associated with survival. Cox proportional hazard model was used to evaluate relative risk for survival. Statistical significance was established at p<0.05.
Of the 68 patients with septic shock, 20 were female and 48 were male. The mean age of the patients was 60±14 yr, and 40 patients (58.8%) died. The mean SAPS II and SOFA scores were 54±14 and 12±4, respectively. The mean basal serum cortisol level was 23±14 µg/dL. In total, 48 patients (70.6%) were diagnosed with RAI. Short corticotropin stimulation test was performed at median 16.1 hr (0.2-137.3 hr) after onset of septic shock.
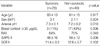
Patient characteristics are shown in Table 1. SAPS II and SOFA scores were significantly different between the RAI and non-RAI groups (p=0.002 and p=0.015, respectively). The lung was the most common site of origin for sepsis in both groups. Bacterial infection was the most common cause of sepsis. The incidence of Gram-positive and Gram-negative bacterial infections was similar in both groups. Malignancy was the most common comorbid illness (54% in RAI group and 40% in the non-RAI group) and refractory shock was the most common cause of death in both groups, with no significant differences between the groups.
The incidence of RAI was 62.5, 79.2, and 70.0% for patients with basal cortisol levels <15, 15-30, and ≥30 µg/dL, respectively (p=0.447) (Table 2). To assess the influence of diurnal variation on the result of short corticotropin stimulation test, the incidence of RAI was analyzed according to the time of corticotropin injection. Eight, 47, and 13 patients, respectively, had the test performed between 0:00-8:00, 8:00-16:00, and 16:00-24:00. The incidence of RAI was 75%, 70%, and 69%, respectively showing no significant difference among three groups (p=0.956). The influence of time lag from the onset of septic shock to the actual start of short corticotropin stimulation test was also assessed. Patients whose time lag was <12 hr, 12-24 hr, 24-48 hr, and >48 hr had RAI in 70% (17/23), 62% (16/26), 75% (9/12), and 86% (6/7) of patients, respectively, showing no statistically significant difference (p=0.566). According to the multivariate analysis with logistic regression, no variable was predictive of RAI (Table 3).
Among patients with RAI, 33 (68.8%) received low-dose corticosteroid replacement therapy (hydrocortisone sodium succinate 50 mg every 6 hr for 7 days). No significant difference in mortality was observed between patients who received corticosteroid replacement therapy and those who did not (73% [11/15] vs. 58% [19/33], p=0.351).
A univariate analysis was performed to compare the characteristics of survivors versus non-survivors (Table 4). A higher SAPS II score (48 in the survival group vs. 58 in the non-survival group, p=0.006) and lower arterial pH (7.3 in the survival group vs. 7.2 in the non-survival group, p=0.010) were significantly associated with hospital mortality, according to the univariate analysis. The survival group demonstrated lower basal serum cortisol levels, although this finding was not significant. Basal serum cortisol levels ≥30 µg/dL were significantly associated with hospital mortality in the univariate analysis (85% mortality in patients with basal serum cortisol ≥30 µg/dL vs. 48% mortality in patients with basal serum cortisol <30 µg/dL, p=0.006). In addition, basal serum cortisol levels ≥30 µg/dL were significantly associated with hospital mortality in the multivariate logistic regression analysis (Table 5). The median time to death was 5 days for patients with basal cortisol levels >30 µg/dL (95% confidence interval [0.84, 9.16]) and 25 days for patients with basal cortisol levels <30 µg/dL (95% confidence interval [10.2, 39.8], p=0.006) (Fig. 1).
Our findings suggest that RAI, diagnosed according to a decreased cortisol response to corticotropin, is frequently observed in Korean patients with septic shock. None of the pretest clinical factors evaluated were predictive of RAI. Although basal cortisol levels were not predictive of response to corticotropin, they still had a prognostic value in patients with septic shock, many of whom had received corticosteroid replacement therapy.
To our knowledge, this is the first study reporting the incidence of RAI in Korean patients with septic shock. The incidence of RAI in septic shock was 70.6%, which is consistent with the findings of Annane et al. (6), demonstrating that RAI is a major clinical problem in Korean patients with septic shock.
Tests such as the random serum cortisol level, short corticotropin stimulation, low-dose short corticotropin stimulation, insulin-induced hypoglycemia, and short metyrapone have been used to diagnose adrenal insufficiency in different patient populations (17). The short corticotropin stimulation test is the most commonly performed test for the diagnosis of primary adrenal insufficiency (18, 19). However, it is limited when hypoadrenalism occurs secondary to recent hypothalamic or pituitary insults and caution is necessary when interpreting the test results (17). Theoretically, evaluation of the integrity of the whole hypothalamic-pituitary-adrenal axis is best served by cortisol response to insulin-induced hypoglycemia (20). However, insulin-induced hypoglycemia may pose risks to patients with ischemic heart disease, epilepsy, or severe cortisol deficiency (10) and may be life-threatening in patients with unstable hemodynamics, such as those with septic shock.
The optimal means of diagnosing RAI in critically ill patients remains controversial. The simplest method is to test a random cortisol level. In an otherwise stable person, a random serum cortisol level ≤3 µg/dL is suggestive of adrenal insufficiency, whereas an adrenal insufficiency may be ruled out with a random cortisol level ≥19 µg/dL (18). However, in critically ill patients, basal serum cortisol levels are usually elevated and correlated with the severity of illness (5), which makes determining an appropriate response difficult. Older studies of RAI have used different levels of cortisol to establish an accurate diagnosis, but none of them have been universally accepted (21).
In this study, we defined RAI as an increase in cortisol level <9 µg/dL from baseline after corticotropin infusion because it has been associated with increased mortality. In addition, corticosteroid replacement therapy has been shown to improve hemodynamics and survival in patients diagnosed with RAI according to this criterion (5, 6, 14), suggesting that it may have clinical utility in diagnosing RAI in patients with septic shock. A 250-µg/dL dose of corticotropin is supraphysiological, and some data suggest that low-dose testing with 1-µg/dL may be more sensitive (22, 23); however, no data are available on the significance of RAI diagnosed with low-dose testing on clinically important endpoints. Notably, a recent study by Marik et al. showed evidence that random serum cortisol levels of <25 µg/dL were more sensitive in predicting hemodynamic responses to hydrocortisone in patients with septic shock than short corticotropin testing using a low or high dose (12). However, in this study, only 22% of patients with septic shock had a decreased response to a standard dose of corticotropin compared to 70.6% in our study and 76.6% in the study by Annane et al. (6). These findings suggest that the patient population studied by Marik et al. was somewhat different from ours and that of Annane et al.
No specific clinical features can help predict RAI in critically ill patients (17, 24). Although symptoms such as fatigue, weight loss, nausea, abdominal pain, arthralgia, and postural syncope are known to be associated with hypoadrenalism, these are nonspecific and frequently found in critically ill patients (10). Similarly, classic features of addisonian crisis, such as anorexia, nausea, vomiting, diarrhea, delirium, fever, and hypotension, are common in sepsis patients (10). Hemodynamic instability despite adequate fluid resuscitation and ongoing evidence of inflammation without an obvious source that does not respond to empirical treatment are also suggestive of corticosteroid insufficiency in critically ill patients (25, 26). However, these are also common features in patients with severe sepsis/septic shock irrespective of the presence of RAI. Thus, it is extremely difficult to recognize adrenal insufficiency in patients who are under intensive care. In the present study, none of the pretest clinical characteristics evaluated were predictive of RAI. SAPS II and SOFA scores were significantly higher in the RAI group compared to the non-RAI according to the univariate analysis; however, the multivariate analysis suggested that these differences were not significant, although the SAPS II score had a borderline significance (p=0.074). The utility of the SAPS II score in predicting RAI warrants further studies.
We evaluated whether a random serum cortisol level was predictive of serum cortisol response to corticotropin. The incidence of RAI, diagnosed as an increase in serum cortisol level <9 µg/dL measured 30-60 min after corticotropin infusion, was similar among all patients irrespective of the basal cortisol level. Even in patients with the highest random serum cortisol levels (≥30 µg/dL), a similar proportion of patients was observed as having a decreased cortisol response to corticotropin (70%). These data suggest that even when serum cortisol is at a high level, patients may not retain the capability to further increase its secretion in response to physiologic needs.
The highest levels of cortisol in critically ill patients have been reported in those with the highest illness severity scores and those with the highest mortality rates (27, 28). Moreover, serum cortisol level has been shown to be an independent predictor of outcome (5, 29). The prognostic importance of a high serum cortisol level may be different in patients receiving corticosteroid replacement therapy due to the influence of the corticosteroid drug. Data from our study population, in which more than two-thirds of patients received corticosteroid replacement therapy, suggest that a random cortisol level >30 µg/dL holds prognostic significance.
The present study had several limitations. Not all of the patients with septic shock underwent short corticotropin stimulation testing during the study period. However, the incidence of RAI (70.6%) and patient characteristics, such as mean age, gender distribution, SAPS II score, basal cortisol level, site of infection, and isolated organisms, were consistent with previous studies (5, 6). Second, not all of the patients with RAI received treatment with corticosteroid replacement therapy, as the decision to administer such treatment was made by the attending physician. Thus, whether corticosteroid replacement therapy improved outcomes in these patients is beyond the scope of this study. But, it may be one of the reasons why there was no significant mortality difference between patients with and without RAI as had been shown by a previous study (5). Third, since daily management of patients was done by different attending physicians, different management strategies of individual septic shock patient may have influenced the outcome of these patients. The patients were managed according to general principles compatible with the "Surviving Sepsis Guideline" which include early hemodynamic resuscitation, antibiotics therapy, drainage of septic focus, and use of vasopressor and an intensivist was available everyday to provide guidance in general critical care (30). But we cannot rule out the possibility that these differences might have contributed to the results of this study including the fact that SAPSII was not a prognostic factor on multivariate analysis.
In conclusion, none of the clinical characteristics we evaluated, including basal serum cortisol level, were predictive of serum cortisol response to corticotropin. However, a high basal cortisol level demonstrated prognostic significance in patients with septic shock.
Figures and Tables
Table 1
Clinical data, sites of sepsis, and bacterial strains diagnosed at the onset of septic shock

RAI, relative adrenal insufficiency; SAPS II, simplified acute physiology score II; SOFA, sequential organ failure assessment; G(+), Gram-positive bacteria; G(-), Gram-negative bacteria; HTN, hypertension; IHD, ischemic heart disease; CHF, congestive heart failure; DM, diabetes mellitus; CRF, chronic renal failure; CNS, central nervous system. Values are expressed as the mean±SD.
References
1. Schein RM, Sprung CL, Marcial E, Napolitano L, Chernow B. Plasma cortisol levels in patients with septic shock. Crit Care Med. 1990. 18:259–263.

2. Besse JC, Bass AD. Potentiation by hydrocortisone of responses to catecholamines in vascular smooth muscle. J Pharmacol Exp Ther. 1966. 154:224–238.
3. Iversen LL, Salt PJ. Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol. 1970. 40:528–530.

4. Kalsner S. Mechanism of hydrocortisone potentiation of responses to epinephrine and norepinephrine in rabbit aorta. Circ Res. 1969. 24:383–395.

5. Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000. 283:1038–1045.

6. Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002. 288:862–871.

7. Sibbald WJ, Short A, Cohen MP, Wilson RF. Variations in adrenocortical responsiveness during severe bacterial infections. Unrecognized adrenocortical insufficiency in severe bacterial infections. Ann Surg. 1977. 186:29–33.
8. Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004. 350:1629–1638.

9. Molijn GJ, Spek JJ, van Uffelen JC, de Jong FH, Brinkmann AO, Bruining HA, Lamberts SW, Koper JW. Differential adaptation of glucocorticoid sensitivity of peripheral blood mononuclear leukocytes in patients with sepsis or septic shock. J Clin Endocrinol Metab. 1995. 80:1799–1803.

10. Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003. 348:727–734.

11. Manglik S, Flores E, Lubarsky L, Fernandez F, Chhibber VL, Tayek JA. Glucocorticoid insufficiency in patients who present to the hospital with severe sepsis: a prospective clinical trial. Crit Care Med. 2003. 31:1668–1675.

12. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003. 31:141–145.

13. Salluh JI, Verdeal JC, Mello GW, Araujo LV, Martins GA, de Sousa Santino M, Soares M. Cortisol levels in patients with severe community-acquired pneumonia. Intensive Care Med. 2006. 32:595–598.

14. Annane D, Bellissant E, Sebille V, Lesieur O, Mathieu B, Raphael JC, Gajdos P. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenal function reserve. Br J Clin Pharmacol. 1998. 46:589–597.

15. Hoen S, Asehnoune K, Brailly-Tabard S, Mazoit JX, Benhamou D, Moine P, Edouard AR. Cortisol response to corticotropin stimulation in trauma patients: influence of hemorrhagic shock. Anesthesiology. 2002. 97:807–813.
16. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992. 20:864–874.
18. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994. 79:923–931.

19. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison's disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992. 75:259–264.

20. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998. 83:2350–2354.

21. Beishuizen A, Thijs LG. Relative adrenal failure in intensive care: an identifiable problem requiring treatment? Best Pract Res Clin Endocrinol Metab. 2001. 15:513–531.

22. Oelkers W. Dose-response aspects in the clinical assessment of the hypothalamo-pituitary-adrenal axis, and the low-dose adrenocorticotropin test. Eur J Endocrinol. 1996. 135:27–33.

23. Zaloga GP, Marik P. Hypothalamic-pituitary-adrenal insufficiency. Crit Care Clin. 2001. 17:25–41.

25. Burchard K. A review of the adrenal cortex and severe inflammation: quest of the "eucorticoid" state. J Trauma. 2001. 51:800–814.

26. Lamberts SW, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. N Engl J Med. 1997. 337:1285–1292.

27. Jurney TH, Cockrell JL Jr, Lindberg JS, Lamiell JM, Wade CE. Spectrum of serum cortisol response to ACTH in ICU patients. Correlation with degree of illness and mortality. Chest. 1987. 92:292–295.
28. Wade CE, Lindberg JS, Cockrell JL, Lamiell JM, Hunt MM, Ducey J, Jurney TH. Upon-admission adrenal steroidogenesis is adapted to the degree of illness in intensive care unit patients. J Clin Endocrinol Metab. 1988. 67:223–227.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download