Abstract
The bioactive sphingolipid metabolite sphingosine 1-phosphate (S1P), recently was reported to induce apoptosis of some cancer cells and neurons, although it generally known to exert mitogenic and antiapoptotic effects. In this study, we investigated the effects of S1P on the cell growth, melanogenesis, and apoptosis of cultured B16 mouse melanoma cells. In results, S1P was found to induce apoptosis in B16 melanoma cells in a dose- and time-dependent manner, but exerted minimal effects on melanogenesis. Although receptors of sphingosine 1-phosphate (endothelial differentiation gene 1 [Edg]/S1P1, Edg5/S1P2, Edg3/S1P3) were expressed in B16 melanoma cells, they were shown not to be associated with S1P-induced apoptosis. In addition, pertussis toxin did not block the apoptotic effects of S1P on B16 melanoma cells. S1P induced caspase-3 activation and the extracellular signal-regulated kinase (ERK) activation. Interestingly, the ERK pathway inhibitor, UO126, reversed the apoptotic effects of S1P on B16 melanoma cells. These results suggest that S1P induced apoptosis of B16 melanoma cells via an Edg receptor-independent, pertussis toxin-insensitive pathway, and appears to be associated with the ERK and caspase-3 activation.
Recent studies suggest that sphingolipids, in addition to their function as structural cell membrane constituents, play a key role as signaling molecules. In this regard, sphingolipid metabolites, including ceramide, sphingosine, and sphingosine 1-phosphate (S1P), have been identified as a new class of lipid messengers, which regulate cell proliferation, differentiation, and survival (1-3). S1P generated by sphingosine kinase, an intracellular enzyme and has been associated with the regulation of cell proliferation, differentiation, survival, and motility (1-5). In contrast to the general association of ceramide and sphingosine with cell growth arrest and apoptosis, S1P that is a downstream metabolite of ceramide, generally known to exert mitogenic and antiapoptotic effects (1-4). One of the better-known sources of S1P are human platelets, from which S1P is released upon activation by physiological stimuli, thereby suggesting that S1P is a factor significantly involved in endothelial injury, inflammation, thrombosis, angiogenesis, and wound healing via an induced increase in the migration and proliferation of endothelial cells (6, 7). S1P reaches levels of up to 0.2 µM in normal plasma, and may be as high as 10 µM in normal serum, presumably reflecting major contributions from platelets (8).
More recently, S1P has been identified as a first messenger, owing to its ability to bind and activate a series of G-protein coupled receptors (GPCRs) located on the cell surface (9), which have been designated S1P1 (endothelial differentiation gene, Edg 1), S1P2 (Edg 5), S1P3 (Edg 3), S1P4 (Edg 6), S1P5 (Edg 8), by a nomenclature subcommittee of the International Union of Pharmacologists (10, 11). S1P can function in either an autocrine or paracrine fashion via Edg receptors (9). The coupling of Edg receptors to diverse G proteins results in the activation of a number of downstream signaling pathways. Intracellular signaling pathways activated by Edg receptors include adenyl cyclase, phospholipase C (PLC), Ras, mitogen-activated protein kinase (MAPK), Rho, and several protein tyrosine kinases (12-18). S1P also acts as an intracellular second messenger to regulate calcium homeostasis, survival and growth (12). Indeed, a variety of effects were observed in conjunction with S1P, apparently depending on the cell type. Recently, S1P has occasionally been associated with certain growth-inhibitory and apoptotic effects in human hepatoma cells, ovarian cancer cells, cultured hippocampal neurons, and others (19-24).
In the present study, we have identified S1P receptors (Edg-1, -3, and -5) in B16 mouse melanoma cells, and investigated the actions of S1P on cell growth. S1P evidenced an antiproliferative effect on B16 mouse melanoma cells. S1P induced apoptosis via caspase-3 activation, in an Edg receptor-independent fashion. S1P activated the extracellular signal-regulated protein kinase (ERK) and UO126, the ERK pathway inhibitor, reversed the apoptotic effects of S1P.
Sphingosine 1-phosphate and dihydrosphingosine 1-phosphate were obtained from Calbiochem (SanDiego, CA, U.S.A.). Stock sphingolipid solutions were dissolved in methanol (S1P and dihydro-S1P). All stock solutions were maintained at -80℃ until use. Dilutions of S1P and dihydro-S1P were freshly prepared after the evaporation of methanol via resuspension in 0.4% fatty acid-free bovine serum albumin (BSA) and sonication. UO126 and pertussis toxin were purchased from the Sigma Co (Yongin, Korea). Stock UO126 solutions were dissolved in Me2SO and maintained at -80℃ until use. Culture media and reagents were obtained from Gibco (LA, CA, U.S.A.), and fetal bovine serum (FBS) was obtained from Omega (Tarzana, CA, U.S.A.). Mouse monoclonal antibody against Edg-3, Edg-5 and rabbit polyclonal antibody against Edg-1 were from Merk Korea (Seoul, Korea). Rabbit monoclonal anti-cleaved caspase-3 (Asp175) antibody was purchased from Cell Signaling Technology, Inc (Danvers, MA, U.S.A.). The ApopTag Kit (S7101) was purchased from the Intergen Company (Norcross, Eeorgia, U.S.A.).
B16 mouse melanoma cells (2×104) were seeded into 30-mm culture dishes in maintenance medium (RPMI 1640 supplemented with 10% FBS, 1% streptomycin and penicillin), and permitted to attach overnight. The medium was then exchanged with serum-starved medium (0.25% FBS) for 48 hr, and treated with S1P or vehicle (PBS/BSA) for the indicated time courses. Every 2 days, the medium was changed.
Cells (1×104 cells/well), seeded into 12-well plates for 24 hr in maintenance media and 48 hr for serum-starvation media, and were incubated with the test substances for 24 hr at 37℃ in 5%CO2. After the addition of 100 µL/well of MTT solution (5 mg/mL), the plates were incubated for an additional 4 hr. The supernatants were then removed, and the formazan crystals were solubilized in 1 mL of dimethyl-sulfoxide. Optical density was determined at a wavelength of 540 nm, using an ELISA reader.
For the quantitative analysis of melanin content, cells were harvested and counted (2×105), and then centrifuged for 4 min with 100,000 r.p.m. After vortex, cells were incubated in 1N of NaOH to lysis overnight at 37℃ and then optical density was determined at 470 nm using an ELISA reader.
Cells were grown for 24 hr on Lab-Tek chamber slides (Nunc Inc., Naperville, IL, U.S.A.). The cells were then fixed in 4% paraformaldehyde containing 0.1% Triton X-100 overnight at 4℃. Nonspecific binding sites were blocked with blocking solution (20% normal goat serum, 0.1% BSA, and 0.1% Triton X-100 in phosphate buffered solution [PBS]) for 10 min at room temperature. The cells were then incubated for 25 min at 45℃ with mouse monoclonal antibody against Edg-3, Edg-5 diluted to 1:50, and rabbit polyclonal antibody against Edg-1 diluted to 1:100, in PBS. After washing with PBS, the cells were incubated with biotin-labeled mouse anti-IgG antibody (1:200, DAKO, Carpinteria, CA, U.S.A.) and streptavidin alkaline phosphate anti-alkaline phosphate, for 15 min each at room temperature. The substrate chromogen New fuchsin (ScyTek, Logan, UT, U.S.A.) was also applied for 20 min. The cells were examined with a Zeiss microscope. PBS instead of a primary antibody was administered as a negative control.
Cells were grown on Lab-Tek chamber slides (Nunc Inc.) for 24 hr in maintenance medium to attach, after which the medium was changed to fresh maintenance medium or serum-starved medium (0.5% FBS) for 48 hr, then treated for 24 hr with S1P or a vehicle. The nick-end-labeling (terminal deoxynucleotidyl transferase-mediated dUTP TUNEL) technique was conducted as described. In brief, the slides were fixed for 10 min in 1% paraformaldehyde in PBS, and then washed twice in PBS. The cells were preincubated for 2 min at room temperature in equilibration buffer, and incubated for 1 hr in a moisture chamber at 37℃ with a working strength (55 µL/5 cm2) formulation of TdT enzyme (77 µL of reaction buffer with 33 µL of TdT enzyme). The reaction was halted via the transference of the slides to stop buffer (1 mL buffer with 34 mL D/W) in a coplin jar, with 15 sec of agitation, incubated for 10 min at room temperature, and then washed three times in PBS. The cells were incubated for 30 min in a moisture chamber at room temperature in anti-dioxigenin peroxidase conjugate, and then washed four times in PBS. DAB peroxidase substrate was applied to the stains for 10 min at room temperature, followed by washing in distilled water. The cells were counterstained with 0.5% methyl green in a coplin jar for 10 min at room temperature, then mounted in an aqueous mounting medium (Niomeda, Foster City, CA, U.S.A.).
We utilized computerized image analysis to quantify the number of apoptotic cells in each sample. A CCD camera (CCD-IRIS, Sony, Tokyo, Japan) mounted on a microscope (Olympus BX50F, Olympus Optical Co., Tokyo) was connected to an IBM personal computer (PC). The image signals acquired by the PC were then evaluated using Image Pro Plus, Version 3.0 (Media Cybernetics Co., Silver Spring, MD, U.S.A.). Image analyses were conducted on four representative areas of the slides, under constant magnification (×200), after which the percentage of apoptotic cells was determined.
Cells were grown for 24 hr in 100 mm culture dishes, subjected to serum starvation for 48 hr, and treated with S1P or vehicle for the indicated times. They were then harvested and lysed in radioimmunoprecipitation (RIPA) buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, and 50 nM Tris [pH 8.0]) containing the phosphatase inhibitor Na3VO4 (10 mM), and the protease inhibitors phenylmethylsulfonyl fluoride (200 mM) and aprotinin (10 µg/mL). Ten to 20 micrograms of protein per lane was separated via SDS-polyacrylamide gel electrophoresis, and blotted onto nitrocellulose membranes, which were then saturated with 2% bovine serum albumin in Tris-buffered saline containing 0.05% Tween 20. The blots were then incubated with the designated primary antibodies at a 1:1,000 dilution, and then incubated further with horseradish peroxidase-conjugated secondary antibody. The bound antibodies were detected using a Renaissance chemiluminescence kit (Dupont NEN, Boston, MA, U.S.A.).
S1P was found to inhibit the proliferation of B16 melanoma cells (Fig. 1). After 24 hr, S1P significantly inhibited cell growth at concentrations in excess of 5 µM (p<0.05). Indeed, after 24 hr, S1P induced the shrinkage, rounding and detachment of cells, whereas serum deprivation alone had no detectable effects on cell morphology (data not shown). To assess the effect of S1P on melanogenesis in B16 melanoma cells, melanin content was measured as described in method. The melanin synthesis of B16 melanoma cells was not significantly affected by S1P (p>0.05, Fig. 2).
In order to identify more precisely the inhibitory effects of S1P on the growth of B16 melanoma cells, we attempted to determine whether S1P induced apoptosis in B16 melanoma cells. Using TUNEL assays, the numbers of positively stained cells were found to have increased after 72 hr of treatment with 10 µM of S1P, whereas the majority of serum-deprived control cells evidenced negative findings (Fig. 3). This was quantitatively analyzed as described in the methods, and the percentage of apoptotic cells after 24 hr of treatment with 10 µM of S1P was found to have been increased significantly as compared with the control group (p<0.001) (Table 1).
In order to further characterize the apoptotic effects of S1P on B16 melanoma cells, we conducted Western blot analysis for caspase-3 activation. As shown in Fig. 4, 10 µM of S1P induced time-dependent caspase-3 activation.
To identify the receptors of sphingosine 1-phosphate on the surfaces of B16 melanoma cells, we conducted immunocytochemical staining with anti Edg-1, -3, and -5, as described in the methods. The plasma membrane of B16 melanoma cells stained positively for Edg-1, Edg-3, and Edg-5 (Fig. 5A). These expressions were verified via Western blot analysis (Fig. 5B).
In order to determine whether S1P triggers apoptosis via Edg receptors, we employed dihydro-S1P (Di-S1P) as the method of Van Brocklyn et al. (15), a structural analogue of S1P that binds and activates Edg receptors, and exerts only receptor-associated signals. 10 µM of Di-S1P induced a slight increase in B16 melanoma cell viability, but exhibited no significant effects in the MTT dye reduction assay (p>0.05) (Fig. 6). This result was contrasted with the apoptotic effects evidenced by S1P. Furthermore, pertussis toxin (PTX), which inhibits cAMP pathway, did not block the apoptotic effects of S1P on B16 melanoma cells (p>0.05) (Fig. 7). These results indicated that the apoptotic effects of S1P occurred via a PTX-insensitive, Edg-independent pathway.
The antiapoptotic functions of ERK, which are known to be activated via Edg receptors, have been well established, and S1P was reported to exert protective effects against apoptosis via ERK and Akt activation in the melanocytes (25). In order to characterize the survival mechanisms by which S1P counteracts its own apoptotic effects, we investigated the activation of ERK by S1P and its responsive role, using UO126, the MEK inhibitor that is a kinase upstream of ERK. According to our results, S1P induced more intense ERK activation than control or Di-S1P until 12 hr, and UO126 blocked this effect (Fig. 8). Interestingly, UO126 pretreatment reversed S1P-induced cell death (Fig. 9, p<0.05). However, UO126 did not work at 24 hr. These results suggested that S1P induced cell death via ERK activation, at least during the early stages.
We determined that sphingosine 1-phosphate induced apoptosis in B16 melanoma cells. The evidence for S1P-induced apoptosis are as follows; first, 24 hr of treatment with more than 5 µM of S1P resulted in a significant inhibition of B16 melanoma cell growth, second, S1P-treated B16 melanoma cells exhibited round, fragmented and condensed nuclei when stained with TUNEL method, and finally, S1P treatment resulted in a stimulation of caspase-3 cleavage, followed by a loss of cell viability. We observed that B16 melanoma cells expressed the Edg receptors of S1P, but the apoptotic effects of S1P were found not to be dependent on the activation of the receptors, and were not blocked by treatment with pertussis toxin. S1P was shown to induce activation of the ERK, and UO126, the MEK inhibitor that is a kinase upstream of the ERK, reversed B16 melanoma cell death induced by S1P. Collectively, our results suggest that S1P induces apoptosis involving the activation of the ERK and caspase, via an Edg receptor-independent and pertussis toxin-insensitive pathway.
This is, to our knowledge, the first study to report that S1P induces apoptosis in B16 melanoma cells. The exact mechanism underlying the apoptotic effects of S1P remains to be elucidated. According to our results, the pathway is Edg-independent, and this is consistent with other reports that reveal the proapoptotic effects of S1P. Recent research into the apoptotic effects of S1P in mesangial cells showed that they were Edg-independent, and the results of this research essentially suggested that the conversion of S1P into sphingosine constituted the relevant mechanism (24). However, the conversion of S1P into sphingosine was not relevant in our study, because S1P-induced apoptosis was caspase-dependent, whereas the cytotoxic effects of sphingosine occurred in a caspase-independent manner (26). In addition, both sphingolipids required similarly high concentrations for the induction of cell death, which militates against the possibility that micromolar concentrations of S1P are being converted completely to micromolar concentrations of sphingosine (27). Davaille et al. (27) suggested that the effects of S1P were concentration-dependent. Whereas micromolar concentrations of S1P exerted proapoptotic effects, which were attributed to an Edg-independent mechanism, submicromolar S1P concentrations evidenced antiapoptotic, proliferative effects via Edg-receptors, and ERK and PI3K/Akt stimulation in hepatic myofibroblasts. Liu et al. (28) showed that sphingosine kinase type 2 was a putative BH3-only protein that induced apoptosis, whereas sphingosine kinase type 1 stimulated both growth and survival. Sphingosine kinase type 2-induced apoptosis was found to be preceded by caspase-3 activation, and occurred independently of Edg receptor activation. This enzyme was also over-expressed under serum starvation conditions. Down regulation of gene of S1P phosphates 1, which dephosphorylates S1P selectively, was detected in stress conditions in NIH3T3 cells (29). Indeed, S1P itself is known to stimulate sphingosine kinase (9). In hippocampal neurons or ovarian cancer cells, S1P-triggered apoptosis relies on a calcium-dependent pathway (20, 21). S1P is capable of mobilizing calcium in the ER, in the absence of plasma membrane S1P receptors (30). Collecting the above findings, S1P is shown to exert proapoptotic signal via Edg-independent intracellular pathway, which may activate calcium or sphingosin kinase type 2 and caspase cascade. It is possible that sphingosin kinase type 2 was over expressed in our study, because we deprived serum from culture media to devoid the effect of S1P existing serum.
By the way, it is interesting the role of the ERK in apoptosis in our study, since the ERK is well known to mediate mitogenic and cell survival effects. However, there are reports that ERK can be associated with apoptosis in certain conditions. In neuronal cells, stimuli that induce prolonged ERK activation, most notably oxidative stress or ischemia, also trigger cell death (31, 32). Transient ERK activation stimulates cell proliferation, but prolonged ERK pathway activation results in cell cycle arrest. However, the mediators of cell death remain enigmatic. Recently, Cagnol et al. (33) showed that caspase-8 was the substrate of prolonged activated ERK in HEK293 cells. In our study, S1P was shown to induce the ERK activation proceeding to the activation of caspase-3 in B16 melanoma cells. This suggested that S1P activates the ERK and then caspases, leading cell death. We are currently undertaking a series of S1P-induced caspases cascade and Bcl-2/Bax protein changes. In melanoma cell lines, Bcl-2 protein is highly expressed to resist apoptosis. Recent study showed Bcl-2 over expression stimulated sphingosine kinase type 1 in human melanoma cell line (34).
In conclusion, sphingosine 1-phosphate exerts apoptotic effects on B16 melanoma cells, an effect which is mediated by ERK and caspase-3 activation. It is a PTX-insensitive and Edg receptor-independent pathway. The downstream pathways associated with the apoptotic effects of S1P should be further investigated. Furthermore, the finding that high dose of S1P can be proapoptotic should be proven also in a couple of other (human) melanoma cell lines. Then it would become clear, whether S1P-induced apoptosis is general for melanoma cells or it is a special feature of B16 melanoma cells.
Figures and Tables
Fig. 1
Treatment with more than 5 µM inhibited the growth of B16 melanoma cells (*: p<0.05). The control was treated with vehicle instead of S1P for 24 hr. Optical density was determined at a wavelength of 540 nm. The experiment was repeated five times.

Fig. 3
The number of apoptotic B16 melanoma cells increased in the S1P treatment group (10 µM for 24 hr) (B) as compared to the vehicle-treated control group (A). (TUNEL, ×200), (Inset: apoptotic cells, ×400).

Fig. 4
Western blot analysis of the cell extracts from B16 cells treated with 10 µM S1P using caspase-3 antibody showed decreased inactive casapse-3 (35 kDa) and increased active cleaved caspase-3 (17, 19 kDa).
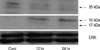
Fig. 5
(A) Cell surface receptors of S1P are expressed abundantly in B16 melanoma cells (Immunocytochemistry, ×400). (B) Western blot analysis of cell extracts from B16 melanoma cells using specific anti-Edg antibodies shows 45 kDa proteins.
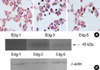
Fig. 6
MTT dye reduction assays were conducted as described in the methods section (Optical density at 540 nm). 24 hr of treatment with dihydro-S1P (10 µM), a structural analogue of S1P that binds and activates only Edg receptors, dose not induce cell death in contrast to the effect of S1P (*, p<0.05). This result suggested that the apoptotic effects of S1P were Edg-independent. This experiment was repeated three times.
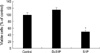
Fig. 7
Serum-deprived cells were preincubated for 24 hr with 10 ng/mL of pertussis toxin (PTX) or vehicle, and incubated further in the presence of PTX or vehicle, together with S1P (10 µM). The MTT dye reduction assays were conducted as was described in the methods section. After time, cell viability was found to have been significantly decreased after treatment with S1P, with or without PTX (*: p<0.05). PTX failed to reverse the apoptotic effects of S1P. This experiment was repeated three times.
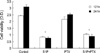
Fig. 8
Serum-deprived cells were preincubated for 1 hr with 10 µM of UO126 or vehicle, and incubated further in the presence of UO126 (the MAP kinase inhibitor) or vehicle, together with 10 µM of S1P or dihydro-S1P, for the indicated time periods. Western blot analysis was conducted as described in the methods section. This figure indicates that S1P activates ERK, and UO126 inhibits the activation of ERK.
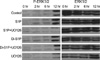
Fig. 9
Serum-deprived cells were preincubated for 1 hr with 10 µM of UO126 or vehicle, and incubated further in the presence of UO126 or vehicle, together with 10 µM of S1P, for the indicated time periods. MTT dye reduction assays were conducted as described in the methods section. After 12 hr, S1P significantly induced cell death, and UO126 reversed S1P-induced cell death (*, p<0.05). This result indicated that S1P induced cell death via ERK activation in early stages. This experiment was repeated three times.

ACKNOWLEDGEMENT
We thank Mr. Young-Bae Kim (Ajou University Hospital, Suwon, Korea) and Ms. Myung-Hee Cha (Inha University Hospital, Incheon, Korea) for technical assistances.
References
1. Sigal YJ, McDermott MI, Morris AJ. Integral membrane lipid phosphatases/ phosphotransferases: common structure and diverse functions. Biochem J. 2005. 387:281–293.
2. Spiegel S, Milstein S. Sphingosine 1-phosphate, signaling inside and out. FEBS Lett. 2000. 476:55–57.
3. Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000. 349:385–402.

4. Spiegel S, Cuvillier O, Edsall LC, Kohama T, Menzeleev R, Olah Z, Olivera A, Pirianov G, Thomas DM, Tu Z, Van Brocklyn JR, Wang F. Sphingosine 1-phosphate in cell growth and cell death. Ann N Y Acad Sci. 1998. 845:11–18.
5. Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998. 12:1589–1598.

6. Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo). 1997. 121:969–973.

7. Ruwisch L, Schafer-Korting M, Kleuser B. An improved high-performance liquid chromatographic method for the determination of sphingosine-1-phosphate in complex biological materials. Naunyn Schmiedebergs Arch Pharmacol. 2001. 363:358–363.

8. Tokumura A. A family of phospholipid autacoids: occurrence, metabolism, and bioactions. Prog Lipid Res. 1995. 34:151–184.

9. Spiegel S, Milstein S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002. 277:25851–25854.

10. Fukushima N, Ishii I, Contos JJ, Weiner JA, Chun J. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001. 41:507–534.
11. Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International union of pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002. 54:265–269.

12. An S, Goetzl EJ, Lee H. Signaling mechanisms and molecular characteristics of G protein-coupled receptors for lysophosphatidic acid and sphingosine-1-phosphate. J Cell Biochem Suppl. 1998. 31:147–157.
13. Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998. 279:1552–1555.

14. Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, Shigematsu H, Takuwa Y. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998. 273:27104–27110.
15. Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillie O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998. 142:229–240.
16. Zondag GC, Postma FR, Etten IV, Verlaan I, Moolenaar WH. Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem J. 1998. 330:605–609.

17. Okamoto H, Takuwa N, Yatomi Y, Gonda K, Shigematsu H, Takuwa Y. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun. 1999. 260:203–208.

18. Gonda K, Okamoto H, Takuwa N, Yatomi Y, Okazaki H, Sakurai T, Kimura S, Sillard R, Harii K, Takuwa Y. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem J. 1999. 337:67–75.

19. Hung WC, Chuang LY. Induction of apoptosis by sphingosine-1-phosphate in human hepatoma cells is associated with enhanced expression of bax gene product. Biochem Biophys Res Commun. 1996. 229:11–15.

20. Hong G, Baudhuin LM, Xu Y. Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Lett. 1999. 460:513–518.

21. Moore AN, Kampfl AW, Zhao X, Hayes RL, Dash PK. Sphingosine-1-phosphate induces apoptosis of cultured hippocampal neurons that requires protein phosphatases and activator protein-1 complexes. Neuroscience. 1999. 94:405–415.

22. Van Brocklyn JR, Tu Z, Edsall L, Schmidt RR, Spiegel S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J Biol Chem. 1999. 274:4626–4632.

23. Davaille J, Gallois C, Habib A, Li L, Mallat A, Tao J, Levade T, Lotersztajn S. Antiproliferative properties of sphingosine 1-phosphate in human hepatic myofibroblasts. A cyclooxygenase-2 mediated pathway. J Biol Chem. 2000. 275:34628–34633.
24. Gennero I, Fauvel J, Nieto M, Cariven C, Gaits F, Briand-Mesange F, Chap H, Salles JP. Apoptotic effect of sphingosine 1-phosphate and increased sphingosine 1-phosphate hydrolysis on mesangial cells cultured at low cell density. J Biol Chem. 2002. 277:12724–12734.

25. Kim DS, Hwang ES, Lee JE, Kim SY, Park KC. Sphingosine-1-phosphate promotes mouse melanocyte survival via ERK and Akt activation. Cell Signal. 2003. 15:919–926.

26. Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000. 476:118–123.

27. Davaille J, Li L, Mallat A, Lotersztajn S. Sphingosine-1-phosphate triggers both apoptotic and survival signals for human hepatic myofibroblasts. J Biol Chem. 2002. 277:37323–37330.
28. Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstein S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003. 278:40330–40336.

29. Lee JS, Jung JH, Kim TH, Seo JS. Changes of gene expression in NIH3T3 cells exposed to osmotic and oxidative stresses. Genomics Inform. 2004. 2:67–74.
30. Kim MY, Liang GH, Kim JA, Kim YJ, Oh S, Suh SH. Sphingosine-1-phosphate activates BKCa channels independently of G protein-coupled receptor in human endothelial cells. Am J Physiol Cell Physiol. 2006. 290:C1000–C1008.
31. Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000. 275:12200–12206.

32. Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001. 98:11569–11574.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download