Abstract
Normal human epidermal keratinocytes (NHEK) respond to the autocrine activated extracellular signal-regulated kinase (ERK) signaling pathway, which contributes to the survival of keratinocytes. However, during the condition of calcium-induced differentiation, how the autocrine ERK signaling is regulated and affected is poorly understood. The purpose of this study was to understand and to obtain clues to the possible function of the autocrine ERK activation during the calcium-induced differentiation of NHEK. We demonstrated that the autocrine activated ERK was not interrupted by calcium triggering and that it was sustained for at least one day after changing the medium. We also found that the autocrine ERK activation was associated with the expression of cyclin D1 and the cell cycle regulation at the early stage of calcium triggering by treating the cells with the mitogen-activated protein kinase inhibitor PD98059. However, the PD98059 treatment did not have a significant influence on the expression of involucrin and loricrin. In addition, we demonstrated that autocrine ERK activation was associated with protein kinase C and p38MAPK signaling. We suggest that the activation of autocrine ERK is not interrupted by calcium triggering and it might participate in cell growth during the early stage of calcium-induced differentiation in NHEK.
The epidermis is a stratified, squamous epithelium that mostly consists of keratinocytes. Proliferating keratinocytes in the basal layer of the epidermis are stratified into the cornified cell envelope via a complex differentiation program. Keratinocytes are one of the best biological systems for studying cell differentiation. Calcium has been known to be a major factor for triggering the differentiation of cultured keratinocytes. The balance between the proliferation of the mitotically active keratinocytes in the basal layer and the differentiation of cells in the suprabasal layers is important to maintain the homeostasis of the epidermis (1-3). When this balance is disturbed, the abnormalities that follow may cause tumorigenesis or other disease states. Thus, understanding the intracellular signaling pathways that are associated with the maintenance of this balance has been the goal for many researchers.
Extracellular signal-regulated kinase (ERK) is a member of the well-characterized mitogen-activated protein kinase (MAPK) family, and it is involved in the regulation of cell proliferation and differentiation. ERK is activated by multiple extracellular stimuli, including growth factors and phorbol esters; it induces a variety of signaling molecules that trigger activation of the factors involved in cell proliferation. In addition, the ERK signaling has also been shown to be involved in the differentiation pathways. In erythroid cells, annexin 1 regulates the erythroid differentiation through the ERK signaling pathway (4). For PC12 cells, ERK activation determines cell differentiation by a sustained activation response to nerve growth factor (NGF) as well as phosphatidylinositol 3 kinase (PI3K) signaling (5, 6). Therefore, other signaling molecules, in addition to ERK, are involved in the early stage of cell differentiation (7).
Keratinocytes secrete cytokines, chemokines, and growth factors that are responsible for the self-proliferation and immune response of the skin (8, 9). Autocrine signaling molecules secreted in the keratinocytes include tumor growth factor (TGF)-α, heparin-binding epidermal growth factor (EGF)-like growth factor, amphiregulin, and epiregulin (10-13). These molecules have been shown to transduce signals through the ErbB and ERK signaling pathways. When growth factor deprivation occurs, keratinocytes release amphiregulin as a ligand to the ErbB1 receptor, and this thereby activates ERK signaling for basal proliferation (14, 15). This autocrine ERK activation is one of the reasons for cell survival and migration during starvation (16). Constitutive activation of MAPK increased the growth rate of human keratinocytes and delayed the onset of terminal differentiation (17). In addition, MAPK cooperates to maintain the epidermal stem cell compartment in vitro (18). Previous reports have shown that the autocrine ERK activation via epidermal growth factor receptor (EGFR) is important for the proliferation and survival of cells (19, 20). Although MAPK signaling generally has specific functions in a variety of cell types, it is considered necessary to have a sustained ERK activity for cell proliferation during the regulation of the cell cycle (21). Many researchers have examined the function of ERK in keratinocytes in association with exogenous stimuli such as UV radiation, wound healing, vitamin D, phorbol ester, and cytokines (15, 22-25). During keratinocyte proliferation, growth factors trigger EGFR autophosphorylation, and this activates Ras signaling through Gab1 and its partner SHP-2. This signaling is required for the maintenance of epidermal MAPK activation in the keratinocytes. In addition, the MAPK signaling pathways modulate the IL-1β expression in human keratinocytes (22) and are involved in the re-epithelialization of fetal skin by the AP-1 transcription factor (24).
Despite the ongoing research on the autocrine activation of ERK in terms of the keratinocytes biology, the questions on how the autocrine ERK signaling is regulated and how it contributes during the calcium-induced differentiation of normal human epidermal keratinocytes (NHEK) have not yet been solved.
In this study, we addressed the question whether the autocrine ERK activation was changed by the calcium-induced differentiation of the NHEK. We found that the calcium triggering did not affect the autocrine ERK activation. The autocrine ERK signaling might participate in controlling the cell cycle even under the condition of calcium triggering at the early stage rather than at the late stage.
NHEK were purchased from Well Skin (Seoul, Korea) and then grown in keratinocyte growth medium (KGM, Cambrex Bioproducts, Walkersville, MD, U.S.A.) supplemented with 0.05 mM calcium, bovine pituitary extract (BPE), human epidermal growth factor (hEGF), insulin, hydrocortisone, and GA-1000. They were subcultured at 60-70% confluency into 60 mm dishes for the experiments. To obtain starved quiescent cells, the medium was changed to keratinocyte basal medium (KBM, without growth factors) 24 hr before stimulation. The cells were switched at 50-60% confluency to fresh KBM containing either 0.05 mM calcium (for the basal state) or 1.2 mM calcium (for to induce differentiation), and then they were cultured for the indicated times. For the inhibitor experiments, inhibitors were added to the medium 30 min before the calcium stimulation. All inhibitors were tested for their non-specific cytotoxicity on the cells using the Celltiter-Blue cell viability assay kit (Promega, Madison, WI, U.S.A.).
Rabbit polyclonal anti-ERK and anti-phospho-ERK antibodies were purchased from Cell Signaling Technologies (Beverly, MA, U.S.A.). Mouse monoclonal anti-cyclin D1 and anti-p27 antibodies were purchased from BD Transduction Laboratories (San Diego, CA, U.S.A.), and the anti-loricrin antibodies were purchased from Covance (Richmond, VA, U.S.A.). The anti-involucrin antibody was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). The inhibitors PD98059, GF109203X, LY294002 and SB203580 were purchased from A.G. Scientific Co, Inc. (San Diego, CA, U.S.A.). PD158780, AG825 and GM6001 were purchased from Calbiochem (San Diego, CA, U.S.A.).
For the preparation of the cell lysates, the keratinocytes were washed twice with the cold phosphate-buffered saline (PBS) and then lysed in lysis buffer (20 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and 10% glycerol). Equal amounts of protein (40 µg) were separated by SDS-polyacrylamide gel electrophoresis and then blotted onto polyvinylidene fluoride membranes. The transferred membranes were immunoblotted with primary antibody in blocking buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Tween 20 and 5% nonfat dry milk) and then detected with horseradish peroxidase-coupled second antibody. Detection was performed using an ECL reaction kit (iNtron, Seoul, Korea) according to the manufacturer's instructions.
The EGFR phosphorylation was quantitated using an ELISA kit (Biosource, Camarillo, CA, U.S.A.) according to the manufacturer's instruction. Briefly, each well was incubated with samples diluted 1:10 for 2 hr at room temperature. The wells were washed with wash buffer and then incubated with anti-Human EGFR [pY 1173] for 1 hr at room temperature. After the washing step, anti-rabbit IgG horseradish peroxidase (a 1:100 dilution in dilution buffer) was added and allowed to incubate for 30 min at room temperature. After washing the plate as described above, the stabilized chromogen was added. After 30 min incubation at room temperature in the dark, the color reaction was stopped by adding stop solution, and it was immediately read on the plate reader at a wavelength of 450 nm.
Ras activity was assessed using the Ras activation assay kit (Upstate Biotechnology, Charlottesville, VA, U.S.A.). Briefly, the cells were washed twice with ice-cold PBS and then lysed in 150 mL MLB buffer (125 mM HEPES, pH 7.5, 750 mM, NaCl, 5% Igepal CA-630, 10 mM MgCl2, 5 mM EDTA and 10% glycerol) containing protease and phosphatase inhibitors (Calbiochem, San Diego, CA, U.S.A.). After centrifugation at 14,000 g for 5 min, the cellular lysates containing 100 µg/mL protein were then incubated with 2 µg/µL of the Raf-1 RBD agarose at 4℃ for 45 min. After washing three times with MLB buffer, the presence of the activated Ras (Ras-GTP) was detected by performing Western blotting with using an anti-Ras monoclonal antibody.
To evaluate the DNA content, the cells were fixed with 70% Ethanol after the indicated times and after stimulation with calcium or calcium plus PD98059. The fixed cells were then washed with Phosphate-buffered saline and incubated with propidium iodide for 30 min. The labeled cells were analyzed with a flow cytometer (FACS Vantage™, Becton Dickinson, San Diego, CA, U.S.A.). The cell cycle distribution was analyzed by using ModFit LT software for Win32, (Verity Software House, Inc., Topsham, ME, U.S.A.).
To examine whether the autocrine ERK activation is affected by calcium-induced differentiation in NHEK, we used either 0.05 mM (Fig. 1A) or 1.2 mM (Fig. 1B) extracellular calcium for the basal or differentiated state of the NHEK, respectively. For the starved state, NHEK were incubated in KBM medium for 24 hr before the addition of calcium. As shown in Fig. 1, the autocrine ERK activation of the NHEK was prominent at the zero time, and it disappeared at 15 min and reappeared 1-2 hr after the medium was changed, as was previously reported (14, 15). It is intriguing that this autocrine ERK activation was sustained for at least for 1 day and was not interrupted during the calcium-induced differentiation (Fig. 1B). Therefore, autocrine ERK activation was sustained for at least 1 day after changing the medium regardless of the calcium concentration; it then started to decline after 3 days. This result led us to raise questions about the role of autocrine ERK activation during the calcium-induced differentiation of NHEK.
First, we needed to confirm the effect of calcium on triggering the differentiation of keratinocytes, since the activation was not affected. We examined the expression levels of the cell cycle regulators and the markers for keratinocyte differentiation to assess the progress of differentiation. The cell cycle activator cyclin D1 was expressed for 1 day and then rapidly disappeared thereafter. In contrast, the cell cycle inhibitor p27, which is also induced by keratinocyte differentiation (26), began to appear at 3 days (Fig. 1C). As expected, involucrin was expressed after the addition of calcium, and it was sustained for 5 days; the loricrin expression started at 5 days (Fig. 1C, left panels). For a comparison, we tested the expression of cell cycle regulators and differentiation markers in the condition of basal (0.05 mM) calcium concentration. Generally, it has been known that keratinocytes, in the condition of starvation (deficient of growth factors), undergo growth arrest and express some differentiation markers because other extracellular signal such as contact inhibition, starvation, detachment from matrix, and chemicals are known as differentiation inducers besides calcium. In our result, growth arrest was progressing and some differentiation markers expressed somewhat lately than calcium-induced differentiation like involucrin (Fig. 1C, right panels) and Keratin 10 (data not shown). These results confirmed that the calcium-induced differentiation of the NHEK had progressed along with the growth arrest and the expression of the differentiation markers. Therefore, we confirmed that the autocrine ERK activation was sustained and it was not interrupted, while the differentiation of the keratinocytes induced by calcium progressed.
We examined the possible contribution of autocrine ERK on the differentiation of NHEK by using the MEK inhibitor PD98059, which resulted in the inhibition of ERK phosphorylation. During the early stage of the calcium-induced differentiation, at the time points of both 6 hr and one day after treatment, the expression of cyclin D1 was suppressed (Fig. 2A). Unexpectedly, however, the expression level of cyclin D1 was induced 3 days after treatment (Fig. 2A). By contrast, the expression of p27 was not altered after treatment (Fig. 2A). These phenomena suggested that autocrine ERK activation was associated with the cell cycle regulators for cell proliferation at the early stage, but it was not at the late stage. Next, to address the relationship with keratinocyte differentiation, we examined the expression of involucrin and loricrin after treatment with PD98059. It was interesting that the pretreatment of PD98059 showed no significant effect. At each time point, the involucrin did not respond to the inhibition of MEK; however, the expression of loricrin was slightly increased after treatment with PD98059 at the time point showing the appearance of loricrin (Fig. 2A). This result suggested that the autocrine ERK activation mainly played a role in the regulation of the cell cycle regulator rather than the expression of the differentiation markers.
We used flow cytometric analysis to examine the effect of PD98059 on the cell cycle of the NHEK. We compared the ratio of G0/G1, which means the population of cells entering the S phase, between the cells pretreated with PD98059 and the cells not pretreated with PD98059 plus high calcium concentrations for 1 day or 3 days. As shown in Fig. 2B, the changes in the G0/G1 ratio between the presence and absence of PD98059 were higher at 1 day (-12%) than at 3 days (less than 2%). These results suggested that the autocrine ERK activation controls the cell cycle at the early stage of differentiation. At later stages, autocrine ERK appears not to be associated with cell cycle regulation.
Kansra et al. (15) have suggested that the autocrine ERK activation in the NHEK is triggered by the signaling from the metalloproteinase-mediated release of amphiregulin through the Ras/EGFR signaling pathway. To understand the signaling pathway controlling the autocrine ERK activation during calcium-induced differentiation, we performed experiments using the Ras activation assay with inhibitors and ELISA for the activated EGFR. We chose three inhibitors: PD158780 (receptor tyrosine kinase inhibitor) to inhibit the ErbB1 autophosphorylation, GM6001 to inhibit metalloproteinase, and AG825 to inhibit ErbB2 phosphorylation. Pretreatment with the first 2 inhibitors showed reduced or nearly abolished levels of the autocrine ERK activation both 6 hr and 1 day after calcium treatment; however, AG825 did not show these results at either time points (Fig. 3A). Next, we performed the ELISA assay for EGFR activation using the extracts at 1 day. As shown in Fig. 3B, the ELISA analysis of the phosphorylated EGFR showed that the functional EGF receptor was decreased in a dose dependent manner with the treatment using the inhibitor PD158780; this was observed while the calcium-induced differentiation progressed. The GTP bound Ras was also shown to be reduced on the Ras activity assay using the same extract used in EGFR activation assay (Fig. 3C). These results suggested that the Ras/EGFR signaling pathway also controlled autocrine ERK activation during the early stage of calcium-induced keratinocyte differentiation as well as the keratinocytes without calcium triggering.
Complex signaling pathways are involved in controlling the differentiation of keratinocytes. Therefore, we examined whether the other signaling pathways affect autocrine ERK signaling during the calcium-induced differentiation by using inhibitors. We used the chemicals GF109203X for the inhibition of the protein kinase C (PKC) family, LY294002 for PI3K, and SB203580 for the p38 MAP kinase because these signaling pathways are well known for controlling the calcium-induced differentiation of NHEK. The autocrine ERK activity was not significantly changed after treatment with either GF109203X or LY294002 after 6 hr of incubation (less than 2-fold). However, SB203580 treatment resulted in reduced autocrine ERK activation (Fig. 4A). On the other hand, after 1 day of incubation, both GF109203X and SB 203580 reduced the autocrine ERK activity (more than 3-fold) (Fig. 4B). This result implied that autocrine ERK activity in the normal keratinocytes was associated with the PKC and p38 MAPK signaling pathways during the early stage of calcium-induced differentiation of NHEK.
The EGFR-dependent ERK signaling pathway plays a role in regulating differentiation, neoplastic transformation and directional migration (27), as well as proliferation. NHEK secrete several ligands, in addition to EGF, and they autonomously stimulate cell proliferation through the Ras/Raf/ERK signaling pathways (11, 15, 28). These findings suggested that NHEK could have a survival signaling pathway for coping with detrimental environmental conditions.
We were curious about how the autocrine ERK activation in the keratinocytes works under the condition of calcium-induced differentiation. From these experiments, first, we have shown that autocrine ERK activation was not interrupted by calcium-induced differentiation and this was sustained for at least 1 day regardless of calcium triggering (Fig. 1). Second, in the early stage of differentiation, the suppression of autocrine ERK activation was observed to have an influence on the expression of cyclin D1 and cell growth (Fig. 2). Third, autocrine ERK activation was also controlled by Ras/EGFR signaling and this was associated with PKC and p38 MAPK kinase signaling during calcium stimulation (Fig. 3, 4).
Schmidt et al. (29) have suggested that stimulation of keratinocytes with extracellular calcium resulted in the activation of ERK, which plays a role in the early stage differentiation of keratinocytes. This report would be the first to demonstrate the relationship between ERK activation and the calcium-induced differentiation of keratinocytes. However, there have been some discrepancies in terms of autocrine ERK activation. Based on the results of other studies, for the HaCaT cell line, the immortalized human keratinocytes did not show any strong autocrine ERK activation after growth factor deprivation. Nevertheless, many reports have shown significant autocrine ERK activation in the NHEK, primary human keratinocytes after starvation (14, 15). Thus, we designed these experiments to determine the role of autocrine ERK activation in the NHEK under the condition of calcium-induced differentiation.
In particular, we noted that the autocrine ERK activation has a biphasic pattern in the early evaluations, after changing to fresh medium. The autocrine ERK activation rapidly declined at 15-30 min after medium change, and it reappeared at 1-2 hr. This phenomenon was also observed in previous reports (15, 30). In our investigation, this pattern was only observed at all time points after changing the medium to the fresh medium (data not shown). This profiling was not affected by a calcium increase. We assumed that this biphasic pattern of the autocrine ERK activation was caused by a time laps after changing to fresh medium that did not include secreted EGFR ligands. Because the autocrine ERK activation was triggered by the ligands secreted autonomously by cell itself, the medium change could create the temporary lack of ligands in the medium.
It is known that ERK activation for cell proliferation controls the expression of cyclin D1 in keratinocytes (14). We have found that before stimulation by calcium, the keratinocytes displayed detectable levels of cyclin D1 after 1 day of starvation, but the levels did not decline until 1 day after calcium stimulation. At 3 days, the cyclin D1 disappeared rapidly and p27 began to be expressed (Fig. 1). The expression pattern of cyclin D1 coincided with the activation of autocrine ERK, and this was suppressed by the MEK inhibitor PD98059 (Fig. 2A). Therefore, the autocrine ERK activation for cell survival was working on the regulation of the cyclin D1 expression before substantial differentiation, along with growth arrest, started. It is interesting that cyclin D1 was induced by treatment with PD98059 at the time point of 3 days (Fig. 2A). We presumed that there would be a kind of negative regulatory system for suppressing the autocrine ERK activation at the late stage of calcium-induced differentiation. Further studies will be needed to examine this. The flow cytometric analysis showed that in the G0/G1 state, the cell population was increased by inhibition of autocrine ERK activation at 1 day after the increase of calcium, yet the increase declined at 3 days (Fig. 2B). This meant that autocrine ERK activation might have a function in controlling the cell cycle at the early stage, but not at the late stage of calcium-induced differentiation. Taken together, these results suggest that NHEK need to stay in the cell survival state for certain a period of time after changing the environmental conditions, such as during starvation or calcium-induced differentiation.
As for the differentiation markers, autocrine ERK activation did not have any influence on the expression of involucrin and it had a slightly negative effect on loricrin. A slight inducing effect of PD98059 on the expression of loricrin was shown in the repeated experiments. Of course, we could not rule out the possibility of the fact that it has a different effect on other differentiation markers. However, this result is partially consistent with previous reports showing that ERK has a negative affect on keratinocyte differentiation (31), which also suggests a possible role for autocrine ERK activation on keratinocyte survival during differentiation. Therefore, we presume that the autocrine ERK activation plays a major role related with cell growth or survival at the early stage of calcium-induced differentiation of NHEK, and it is partially involved in the expression of differentiation markers at the late stage.
In conclusion, even though the basic roles of the autocrine ERK activation in keratinocytes have been suggested, the destination of this signaling during the condition of calcium-induced differentiation has not been investigated. Based on our results, we showed that the autocrine ERK activity was not interrupted by calcium triggering and it has an influence on controlling the cell cycle during the early stages of calcium-induced differentiation. The results also provide basic clues that the autocrine ERK activation in normal keratinocytes would be needed to keep the cells in the survival state while changing the cells' condition, such as during cell differentiation. In addition, we are currently performing further experiments to investigate the mechanisms of the regulation of the autocrine ERK activation, including the interactions with the other signaling pathways during the progress of differentiation.
Figures and Tables
Fig. 1
Autocrine extracellular signal-regulated kinase (ERK) activation of normal human epidermal keratinocytes (NHEK) during calcium-induced differentiation. NHEK were grown until 50% confluence in keratinocyte growth medium (KGM) medium. The cells were changed to basal medium (KBM, without growth factors) for 24 h and then transferred to fresh KBM with 0.05 mM calcium (A) or 1.2 mM calcium (B) for the indicated times. Each extract was then assayed for phospho-ERK or the total ERK by performing western blotting. The activated ERK was sustained for 1 day and it was not interrupted by calcium triggering and then started to decline after 3 days. (C) After the lysates were prepared the same way as (A) and (B), the lysates were then subjected to Western blotting with each of the antibodies indicated to the middle of the autoradiograms. For a comparison, we tested the expression of cell cycle regulators and differentiation markers in the conditions of 1.2 mM (left panel) and 0.05 mM (right panel) calcium concentration. Cell cycle regulators, cyclin D1 and p27 were expressed at the early stage and at the late stage of calcium-induced differentiation, respectively. The differentiation markers, involucrin and loricrin, were appropriately expressed.

Fig. 2
The MEK inhibitor, PD98059, suppressed cyclin D1 and increased G1/G0 stage during the early stage of cell differentiation. (A) The autocrine ERK activation was analyzed by pretreatment with PD98059 in the presence of a 1.2 mM calcium concentration. Normal human keratinocytes were starved for 24 hrs and then incubated with calcium (1.2 mM) for the indicated times in the presence or absence of PD 98059 (20 µM). Each extract was prepared at the indicated times and they were assessed for the phospho-ERK, cyclin D1, p27, involucrin and loricrin expressions by performing Western blot assay. The total ERK was used as the indicator for equal amounts of extracts. The autocrine ERK activation was associated with cyclin D1 expression. (B) The cells were prepared as above for 1 and 3 days, and then they were fixed with ethanol and stained with propidium iodide. Their DNA content was determined by flow cytometry. The distribution of each cell cycle is indicated by the percentage and demarcations in each panel. The demarcations indicated the sub G1, G0/G1 and S/G2/M (the third plus the fourth demarcation) by the left to right sequence, respectively. The changes in the G0/G1 ratio between the presence and absence of PD98059 were higher at 1 day (-12%) than at 3 days (less than 2%). The data are representative of three independent experiments and represents the mean value±SEM of three independent experiments (p<0.05).

Fig. 3
Autocrine ERK activation during the calcium-induced differentiation was dependent on the Ras/EGFR signaling. (A) Normal human keratinocytes were starved for 24 hr and then pretreated the inhibitors, GM; GM6001 (40 µM), PD; PD158780 (300 nM), and AG; AG825 (25 µM) for 30 min, and then calcium was added to the medium (1.2 mM). After the lysates were prepared for the indicated times, they were subjected into Western blotting with anti-phospho ERK antibody. The autocrine ERK activation was dependent on the EGFR signaling. (B) The extracts from cells pretreated with 200, 300 and 400 nM concentrations of PD158780, and a control without pretreatment were prepared; all were subjected to ELISA assay for phospho-EGFR as described in the materials and methods section. The incubation time in the presence of calcium was 1 day. The data represents the mean value±SEM of three independent experiments (p<0.05). (C) The same extracts used in the ELISA assay were used for the Ras activity assay. Representative data are presented from three independent experiments.
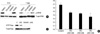
Fig. 4
PKC and p38 MAPK signaling is associated with autocrine ERK activation during the calcium-induced differentiation of NHEK. NHEK were starved for 24 hrs and then they were incubated with calcium (1.2 mM) for 6 hrs (A) and 1 day (B) in the absence or presence of GF; GF109203X (10 µM), LY; LY294002 (10 µM), and SB; SB203580 (10 µM). Each extract was assessed for the expression of phospho-ERK. The total ERK was used to indicate equal amounts of the extracts. The autocrine ERK activation was related with PKC and p38 MAPK signaling during the early stage of differentiation. Each result was analyzed by densitometry and bars represent the mean value normalized to the expression of total ERK, p<0.05, N=3.

References
1. Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocytes. Cell. 1980. 19:1033–1042.
3. Eckert RL. Structure, function, and differentiation of the keratinocytes. Physiol Rev. 1989. 69:1316–1346.
4. Huo XF, Zhang JW. Annexin1 regulates the erythroid differentiation through ERK signaling pathway. Biochem Biophys Res Commun. 2005. 331:1346–1352.

5. Nowroozi N, Raffioni S, Wang T, Apostol BL, Bradshaw RA, Thompson LM. Sustained ERK1/2 but not STAT1 or 3 activation is required for thanatophoric dysplasia phenotypes in PC12 cells. Hum Mol Genet. 2005. 14:1529–1538.

6. Kim Y, Seger R, Suresh Babu CV, Hwang SY, Yoo YS. A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol Cells. 2004. 18:353–359.
7. Puri PL, Wu Z, Zhang P, Wood LD, Bhakta KS, Han J, Feramisco JR, Karin M, Wang JY. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000. 14:574–584.
8. Uchi H, Terao H, Koga T, Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000. 24:29–38.

9. Matsubara M, Harada D, Manabe H, Hasegawa K. Staphylococcus aureus peptidoblycan stimulates GM-CSF production from human epidermal keratinocytes via mitogen-activated protein kinases. FEBS Lett. 2004. 556:195–200.
10. Coffey RJ Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987. 328:817–820.
11. Barnard JA, Graves-Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, Bishop PR, Damstrup L, Coffey RJ. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994. 269:22817–22822.

12. Cribbs RK, Luquette MH, Besner GE. Acceleration of partial-thickness burn wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF). J Burn Care Rehabil. 1998. 19:95–101.

13. Draper BK, Komurasaki T, Davidson MK, Nanney LB. Epiregulin is more potent than EGF or TGFalpha in promoting in vitro wound closure due to enhanced ERK/MAPK activation. J Cell Biochem. 2003. 89:1126–1137.
14. Iordanov MS, Choi RJ, Ryabinina OP, Dinh TH, Bright RK, Magun BE. The UV (Ribotoxic) stress response of human keratinocytes involves the unexpected uncoupling of the Ras-extracellular signalregulated kinase signaling cascade from the activated epidermal growth factor receptor. Mol Cell Biol. 2002. 22:5380–5394.

15. Kansra S, Stoll SW, Johnson JL, Elder JT. Autocrine extracellular signal-regulated kinase (ERK) activation in normal human keratinocytes: metalloproteinase-mediated release of amphiregulin triggers signaling from ErbB1 to ERK. Mol Biol Cell. 2004. 15:4299–4309.

16. Praskova M, Kalenderova S, Miteva L, Poumay Y, Mitev V. Dual role of protein kinase C on mitogen-activated protein kinase activation and human keratinocyte proliferation. Exp Dermatol. 2002. 11:344–348.

17. Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001. 108:527–536.

18. Zhu AJ, Haase I, Watt FM. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci USA. 1999. 96:6728–6733.
19. Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000. 102:211–220.

20. Manohar A, Shome SG, Lamar J, Stirling L, Iyer V, Pumiglia K, DiPersio CM. Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J Cell Sci. 2004. 117:4043–4054.
21. Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001. 11:48–53.

22. Henley DV, Bellone CJ, Williams DA, Ruh MF. MAPK signaling pathways modulate IL-1β expression in human keratinocytes. Arch Biochem Biophys. 2004. 424:112–118.

23. Johansen C, Kragballe K, Henningsen J, Westergaard M, Kristiansen K, Iversen L. 1alpha,25-dihydroxyvitamin D3 stimulates activator protein 1 DNA-binding activity by a phosphatidylinositol 3-kinase/Ras/MEK/extracellular signal regulated kinase 1/2 and c-Jun N-terminal kinase 1-dependent increase in c-Fos, Fra1, and c-Jun expression in human keratinocytes. J Invest Dermatol. 2003. 120:561–570.
24. Gangnuss S, Cowin AJ, Daehn IS, Hatzirodos N, Rothnagel JA, Varelias A, Rayner TE. Regulation of MAPK activation, AP-1 transcription factor expression and keratinocytes differentiation in wounded fetal skin. J Invest Dermatol. 2004. 122:791–804.
25. Gniadecki R. Stimulation versus inhibition of keratinocyte growth by 1,25-Dihydroxyvitamin D3: dependence on cell culture conditions. J Invest Dermatol. 1996. 106:510–516.

26. Harvat BL, Wang A, Seth P, Jetten AM. Up-regulation of p27Kip1, p21WAF1/Cip1 and p16Ink4a is associated with, but not sufficient for, induction of squamous differentiation. J Cell Sci. 1998. 111:1185–1196.

27. Chernyavsky AI, Arredondo J, Karlsson E, Wessler I, Grando SA. The Ras/Raf-1/MEK1/ERK signaling pathway coupled to integrin expression mediates cholinergic regulation of keratinocyte directional migration. J Biol Chem. 2005. 280:39220–39228.

28. Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994. 269:20060–20066.

29. Schmidt M, Goebeler M, Posern G, Feller SM, Seitz CS, Brocker EB, Rapp UR, Ludwig S. Ras-independent activation of the Raf/MEK/ERK pathway upon calcium-induced differentiation of keratinocytes. J Biol Chem. 2000. 275:41011–41017.

30. Seo HR, Kwan YW, Cho CK, Bae S, Lee SJ, Soh JW, Chung HY, Lee YS. PKCalpha induces differentiation through ERK1/2 phosphorylation in mouse keratinocytes. Exp Mol Med. 2004. 36:292–299.
31. Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003. 278:34277–34285.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download