Abstract
Sertoli cells (SC) are known to contain immunoprotective properties, which allow them to survive as allografts without the use of immunosuppressive drugs. Experiments were designed to determine which factors are related to prolonged survival of allogeneic SC. Balb/c derived Sertoli (TM4) and colon cancer (CT-26) cell lines were implanted beneath the kidney capsule of non-immunosuppressed C57BL/6 mice and compared their survival as allografts. Compared to TM4 graft, which survived more than 7 days after transplantation, CT-26 showed massive infiltration of polymorphonuclear cells, necrosis and enlargement of draining lymph nodes. Cultured cell lines showed no differences in their expression patterns of FasL, TGF β1, clusterin and two complement regulatory proteins (CRP, i.e., membrane cofactor protein, MCP; decay accelerating factor, DAF), but protectin (CD59), another member of CRP was expressed only on TM4. These results suggest that CD59 and unknown factors may contribute to the prolonged survival of SC in non-immunoprivileged sites.
Sertoli cells (SC), which constitute the structural framework that support developing germ cells in the seminiferous tubules, have been implicated as the major component of immune privilege in the testis (1). In fact, ectopically transplanted allogeneic SC survived and co-transplantation of allogeneic islets with SC aggregates allowed long-term graft survival without additional systemic immunosuppression in animal models (2, 3). Also xenogeneic SC from neonatal pigs (NPSC) were able to survive cell-mediated rejection when transplanted as xenografts in nonimmunosuppressed rodents (4).
SC are known to express fas ligand (FasL) (5), transforming growth factor (TGF)β1 (6), which are believed to be related with immunoprotective, anti-inflammatory properties respectively. However, high levels of FasL expression does not always correlate with graft survival, as shown in pancreatic islet transplantation models (7). FasL induces polymorphonuclear cell (PMN) activation (8), and the inflammation can be modulated by TGFβ1 (9). But it is not clear whether the expression of FasL and TGFβ1 qualify prolonged allograft survival.
In addition, SC produce clusterin, an amphipathic glycoprotein, which has many anti-inflammatory activities and have been shown to produce many immunoprotective factors (10). As complement plays a dominant role in the process of humoral immunity-mediated rejection, regulation of complement activation may be related to the prolonged graft survival of SC. In fact, complement regulatory protein (CRP) such as membrane cofactor protein (MCP), decay-accelerating factor (DAF) and protectin (CD59) are known to be expressed in the testis (11), but it is not clear whether SC produce CRP, thus providing a microenvironment that may aid allograft survival at non-privileged sites.
Our study was performed to test whether the expression of FasL, TGFβ1, and clusterin qualify prolonged allograft survival and to determine additional factors that may be related to the prolonged survival of SC at non-privileged sites.
C57BL/6 female mice, aged 7 weeks, were purchased form Orient Corporation (Korea) and used as recipients. All animal experiments were performed with approval of the Institutional Animal Care and Use Committee (IACUC) of Clinical Research Institute in Seoul National University Hospital. And National Research Council (NRC) 'Guidelines for the Care and Use of Laboratory Animals' were followed (revised 1996).
Two Balb/c derived cell lines, TM-4 and CT-26 were used as an allogeneic SC and control cells, respectively. The first was established cell lines of non-tumorigenic SC (12) and the latter is colon carcinoma. Both cell lines were obtained from the Korea Cell line Bank (KCLB). TM4 was cultured 37℃, 5% CO2 incubator. Culture medium were 1:1 mixture of Ham's F12 medium and Dulbecco's modified Eagle's Medium supplemented with 4.5 g/L glucose, 1.2 g/L sodium bicarbonate and 15 mM HEPES, horse serum (5%), heat inactivated fetal bovine serum (2.5%). CT-26 was cultured in Dulbecco's modified Eagle's Medium supplemented with heat inactivated fetal bovine serum (10%).
C57BL/6 mice were anesthetized by intraperitoneal injection of ketamine-rumpon mixture and then transplanted with 5×106 cells respectively. Left kidney was exposed via transverse abdominal incisions. Cells for implantation were harvested immediately prior to implantation, and the viability and number of each cell line was determined. Each counted cells were placed in yellow tips (Gilson), closed with paraffin, pelleted by centrifugation, and gently placed under the left renal subcapsular space after removing paraffin.
Nephrectomies were performed for morphologic assessment at post operation day (POD) 2 (n=3), 7 (n=3), and 15 (n=3). The graft-bearing kidneys were immersed in 4% paraformaldehyde solution and embedded in paraffin. After deparaffinization and rehydration, tissue sections were stained hematoxylin and eosin. Coded slides were examined by light microscopy.
For analysis of T cell phenotype changes in draining lymph node, lymphocytes from renal lymph node at 2, 7, 15 posttransplant days were counted and evaluated for the expression of T cell activation markers. Briefly, cells were incubated with various fluorescence-labeled monoclonal antibody (BD Pharmingen, San Diego, CA, U.S.A.) diluted at optimal concentration for immunostaining. Immunoglobin G block (2.4G2) were used to avoid the nonspecific binding to Fc receptors. To determine the intracellular expression of FasL and TGFβ1 on cultured cells, 5×105 nonfixed single cells before transplantation, intracellular staining was performed using Cytofix/Cytoperm™ Plus (BD Pharmingen). Briefly, the cells were harvested and washed twice in PBS, and cytofix/cytoperm was added for 20 min at 4℃. The cells were washed twice in perm/wash and resuspended in 100 µL of perm/wash. Biotinylated rat anti-TGFβ1 or anti-FasL (BD Pharmingen) was added at a dilution of 1:100 to each tube and incubated for 30 min at 4℃. Subsequently washed twice in perm/wash, Streptavidin-Fluorescein Isothiocyanate (BD Pharmingen) was added at a dilution of 1:500 and incubated for 30 min at 4℃. After the final incubation, the cells were washed twice in perm/wash, and the fluorescence was measured on a FACS Caliber (BD Pharmingen).
Total RNA was extracted using EazyBlue (Intron Biotechnology, Inc., Sungnam, Korea). All RNA samples were stored at -80℃ until reverse transcription. Briefly, total RNA (3 µg) was reverse transcribed in 20 µL, using 200 U recombinant M-MLV Reverse Transcriptase (Bioneer Corp., Daejeon, Korea), 20 U RNAsin (Invitrogen Corp., San Diego, CA, U.S.A.), 5 mM each dNTP mix (Intron Biotechnology Inc.), 100 pMol oligo dT18VN (COSMO Co. Ltd, Korea), and 10 mM of dithiothreitol for 1 hr at 42℃. All samples were reverse transcribed using the same master mix to minimize nonspecific differences.
Semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using a DNA thermal cycler (PTC0200 DNA Engine, MJ Research Inc., Waltham, MA, U.S.A.). cDNA (2.5 µL) was amplified in a 50-µL reaction volume containing; 1 mM dNTPs, 2 mM MgCl2, 10 pMol primers, 2.5 U Taq (Intron Biotechnology Inc.), and reaction buffer (supplied with enzyme) using the following conditions; 94℃ (5 min) hot start followed by 35 amplification cycles of; 94℃ (45 sec), 51 to 60℃ (45-60 sec), and 72℃ (45 sec). The nucleotide sequences and annealing temperatures of the primer pairs were as follows: FasL (56.5℃) 5'-GCC CAT GAA TTA CCC ATG TC-3' and 5'-GTT CTG CCA GTT CCT TCT GC-3' (326 bp); TGFβ1 (55℃) 5'-GCT CAC TAC TCT GGA TAC GG-3' and 5'-ATC CAC ACA GAC AAC TTT CC-3' (466 bp); clusterin (55.4℃) 5'-CAG TTC CCA GAC GTG GAT TT-3' and 5'-TGA GGT GTT GAG CAT CTT CG-3' (267 bp) ; MCP (52℃) 5'-GCC CTT CTG TTT CTG CTG TC-3' and 5'-CCA AAC GAA GGG TCC TGT AA-3' (257 bp); DAF (52℃) 5'-CCC ACC TCC AGA CAT TCC TA-3' and 5'-TGG GAG TGG AGG TTG TTT TC-3' (310 bp); CD59 (55℃) 5'-CAG TCA CTG GCG ATC TGA AA-3' and 5'-AGA TTC AAA ATG GCC ACC AG-3' (427 bp); GAPDH (54.3℃) 5'-AAC TTT GGC ATT GTG GAA GG-3' and 5'-ACA CAT TGG GGG TAG GAA CA-3' (223 bp). PCR products were run in 1.5% agarose gels containing ethidium bromide staining (0.5 µg/mL) and photographed under UV light. The sizes of PCR products were verified against 100-bp ladders (Bioneer Corp.).
Cells implanted under the kidney capsule of immunocompetent C57BL/6 mice were determinded macro- and microscopically to ascertain whether they could survive as an allo-geneic grafts. Macroscopically, cellular grafts were easily identifiable at POD 2 and POD 7, but not at POD 15. To further confirm the survival of grafts, tissue sections were examined histologically (Fig. 1, 2). Histological examination of graft-bearing kidneys was performed by hematoxylin-eosin staining to detect the presence of transplanted cells. On a specimen taken at POD 2, abundant grafted cells were detected under the kidney capsule of both groups and overlying the kidney, whereas kidney capsules of CT-26 transplant, harvested at POD 7, revealed an extensive lymphocytic infiltration and central necrosis (Fig. 2C, D). Whereas the composite allografts from animals of TM4 transplant demonstrated a relatively small number of lymphocytes (Fig. 2A, B). At POD 15, there was no implanted cells identified in either groups.
Macroscopically, renal lymph nodes from CT-26 transplant were more distended than TM4 transplant and revealed a time-dependent increase of lymphocyte numbers, 6 times at POD 2, 12 times at POD 7 (data not shown). Flow cytometric analysis for CD4+CD44+ T cell populations in the draining lymph node showed an early increase up to POD 7 and decreased gradually thereafter. CD4+CD62L+ T cells showed a marked increase at POD 15 in both groups. But there were no differences in the percentage of T cell phenotype between two groups (data not shown).
To compare the characteristics of the two cell lines, we evaluated the expression of FasL, TGFβ1 and clusterin using flowcytometry and RT-PCR. As shown in Fig. 3, there were no remarkable differences in the expression of FasL and TGFβ1. RT-PCR analysis for FasL and TGFβ1 paralleded the result of FACS analyses (Fig. 4). The expression of clusterin mRNA was relatively high in CT-26 cells compared to TM4. Although further analysis is required for verification whether the mRNA expression of these molecules are effectively translated into protein during immune attack and secreted as soluble factors, these results suggest that the expression of theses molecules do not qualify the prolonged allogeneic SC survival.
To identify a possible mechanism related to prolonged survival of allogeneic cellular graft, both cell lines were examined for the expression of complement regulatory proteins i.e., MCP, DAF, CD59. Interestingly mRNA of CD46 and DAF were not detected in both cell lines but the expression of CD59 mRNA were detected only in TM4 (Fig. 4). These results suggest that the expression of CD59 may contribute to the allogeneic SC survival.
Our results showed that complement regulatory protein, CD59, may be related to the prolonged survival of allogeneic SC at non-immunoprivileged sites. TM4 showed prolonged survival compared to CT-26 (Fig. 1, 2). TM-4, implanted beneath the kidney capsule of immunocompetent B6 mice, showed negligible histologic changes at least up to 7 days after implantation compared to CT-26, which showed severe PMN infiltration and necrosis at same time points. But these two cell lines showed similar expression of FasL, TGFβ1 and clusterin on FACS analyses and RT-PCR (Fig. 3, 4). Also there was no difference in T cell subpopulations from draining lymph nodes at each time point, and the higher number of lymphocyte from renal lymph node in the CT-26 transplanted group suggests that TM4 may have certain immune-modulating mechanisms that can delay the rejection from host immune response. It has been shown that immune privilege may be maintained partially by the local production and release of immunosuppressive cytokines (13) and neuropeptides (14). In fact, SC are known to secrete unidentified factors that decrease IL-2 production (15) and T cell proliferation (16).
Fas-FasL interactions are known to be an important mechanism for the maintenance of immune privilege and have been implicated as a form of peripheral tolerance (18), but it was not proven as a mediator of the protective effects of SC in our experiment. Previous reports suggested that TGFβ1 from SC modulates cell activity and favors Th2 over Th1 cell differentiation (19). However, considering the same expression of TGFβ1 in both cell line (Fig. 3, 4), this phenomenon might be caused not only by TGFβ1 but also by other unknown factors. Also the concept of immune privilege as defined in terms of expressions of clusterin that prolong the survival of allogeneic grafts were not applied to CT-26.
On the other hand, several soluble factors including neuropeptide such as vasoactive intestinal peptide and alpha melanocyte stimulating hormone and glucocorticoids, have been implicated in the maintenance of immune privilege (20). SC provide numerous factors required for the orderly development and protection of spermatozoa and germ cells from immune attacks by synthesizing and secreting factors that may lead to local immune tolerance (15, 17). It is possible that one of the many functions of SC in testis is to secret complement inhibitors, which coat the spermatozoa and protect them from complement lysis. Humoral immunity-mediated graft rejection occurs when natural antibodies bind alloantigens located on the surface of target cells, resulting in the activation of the enzymatic cascade via the classical pathway leading to formation of the membrane attack complex (MAC) and cellular lysis. This can be overcome by over-expression in the donor, CRP such as MCP, DAF, and CD59. As complement plays a dominant role in the process of humoral immunity-mediated rejection, the regulation of complement activation is required to overcome graft rejection. In the absence of these proteins, C3 convertase of both the classical and alternative pathways as well as MAC would be deposited, leading to graft damage. In fact, inhibition of graft rejection has been achieved using the transgenic approach with varying success rates, depending on the experimental model (21, 22). Our first (to our knowledge) identification of CD59 expression on SC suggests that a regulatory system exists to protect SC from humoral immunity-mediated graft destruction by complement activation.
In summary, our results suggest that expressions of CD59 may contribute to the survival of allogeneic SC and immune privileged microvironment may not be guaranteed just by the expression of FasL, TGFβ1, or clusterin. Further studies on the immunoprotective factors produced by SC may identify novel mechanisms including immune modulatory proteins that may provide clues for promoting local tolerogenic environment.
Figures and Tables
Fig. 1
Photomicrographs of allografts stained with hematoxylin and eosin at POD 2. Abundant grafted cells were detected under the kidney capsule of both groups (A and B, TM4; C and D, CT-26) and overlying the kidney. There are few lymphocyte infiltration (arrows). Original magnification ×40 (A and C) and ×200 (B and D).
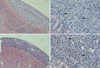
Fig. 2
Photomicrographs of allografts stained with hematoxylin and eosin at POD 7. There are focal infiltration of lymphocytes in TM4 grafts (A and B), whereas the CT-26 graft shows PMN infiltration and central necrosis (C and D). Arrows indicate infiltrated lymphocytes and PMN. Original magnification ×40 (A and C) and ×200 (B and D).
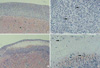
Fig. 3
Expressions of FasL (upper panel) and TGFβ1 (lower panel) in cultured cell lines. FACS analysis shows the similar expression of FasL and TGFβ1. Control analyses (thin line) involve only the fluorescent secondary antibody.

Fig. 4
RT-PCR analysis of mRNA expression in cultured cell lines. Each lane shows the mRNA expression in TM4 (T), CT-26 (C), and control (mouse testis, Ctl). There were no remarkable differences in the expression of FasL, TGFβ1, MCP, and DAF. Clusterin expression shows relatively high in CT-26. Of interest, CD59 mRNA was expressed only in TM4.

References
1. Dufour JM, Rajotte RV, Seeberger K, Kin T, Korbutt GS. Long-term survival of neonatal porcine Sertoli cells in non-immunosuppressed rats. Xenotransplantation. 2003. 10:577–586.

2. Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997. 46:317–322.

3. Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993. 2:123–129.

4. Gores PF, Hayes DH, Copeland MJ, Korbutt GS, Halberstadt C, Kirkpatrick SA, Rajotte RV. Long-term survival of intratesticular porcine islets in nonimmunosuppressed beagles. Transplantation. 2003. 75:613–618.

5. Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995. 377:630–632.

6. Cupp AS, Kim G, Skinner MK. Expression and action of transforming growth factor beta (TGFbeta1, TGFbeta2, and TGFbeta3) during embryonic rat testis development. Biol Reprod. 1999. 60:1304–1313.
7. Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997. 3:738–743.

8. Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L). Science. 1998. 282:1714–1717.

9. Suarez-Pinzon W, Korbutt GS, Power R, Hooton J, Rajotte RV, Rabinovitch A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes. 2000. 49:1810–1818.

10. Dufour JM, Rajotte RV, Korbutt GS, Emerich DF. Harnessing the immunomodulatory properties of Sertoli cells to enable xenotransplantation in type I diabetes. Immunol Invest. 2003. 32:275–297.

11. Perez de la Lastra JM, Harris CL, Hinchliffe SJ, Holt DS, Rushmore NK, Morgan BP. Pigs express multiple forms of decay-accelerating factor (CD55), all of which contain only three short consensus repeats. J Immunol. 2000. 165:2563–2573.

12. Mather JP. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980. 23:243–252.
13. Hooper P, Bora NS, Kaplan HJ, Ferguson TA. Inhibition of lymphocyte proliferation by resident ocular cells. Curr Eye Res. 1991. 10:363–372.

14. Ferguson TA, Fletcher S, Herndon J, Griffith TS. Neuropeptides modulate immune deviation induced via the anterior chamber of the eye. J Immunol. 1995. 155:1746–1756.
15. Selawry HP, Kotb M, Herrod HG, Lu ZN. Production of a factor, or factors, suppressing IL-2 production and T cell proliferation by Sertoli cell-enriched preparations. A potential role for islet transplantation in an immunologically privileged site. Transplantation. 1991. 52:846–850.
16. De Cesaris P, Filippini A, Cervelli C, Riccioli A, Muci S, Starace G, Stefanini M, Ziparo E. Immunosuppressive molecules produced by Sertoli cells cultured in vitro: biological effects on lymphocytes. Biochem Biophys Res Commun. 1992. 186:1639–1646.
17. Korbutt GS, Suarez-Pinzon WL, Power RF, Rajotte RV, Rabinovitch A. Testicular Sertoli cells exert both protective and destructive effects on syngeneic islet grafts in non-obese diabetic mice. Diabetologia. 2000. 43:474–480.

18. Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, Green DR. Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int Immunol. 1995. 7:1451–1458.

19. King C, Davies J, Mueller R, Lee MS, Krahl T, Yeung B, O'Connor E, Sarvetnick N. TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity. 1998. 8:601–613.
20. Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol Rev. 1997. 156:167–184.

21. Thorley BR, Milland J, Christiansen D, Lanteri MB, McInnes B, Moeller I, Rivailler P, Horvat B, Rabourdin-Combe C, Gerlier D, McKenzie IF, Loveland BE. Transgenic expression of a CD46 (membrane cofactor protein) minigene: studies of xenotransplantation and measles virus infection. Eur J Immunol. 1997. 27:726–734.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download