Abstract
Endoscopic submucosal dissection (ESD) has been reported to have a higher bleeding rate than conventional methods. However, there are few reports on whether a proton pump inhibitor or a histamine2-receptor antagonist is the more effective treatment for preventing bleeding after ESD. In a prospective trial, patients undergoing ESD due to gastric adenoma or adenocarcinoma were randomly assigned to pantoprazole or famotidine. Both drugs were given intravenously for the first 2 days, thereafter by mouth. Eighty-five in the pantoprazole group and 79 in the famotidine group were included for analysis. Primary outcome measure was the delayed bleeding rate. Clinical characteristics were not different between the two groups. The delayed bleeding rate was significantly lower in the pantoprazole group compared with the famotidine group (3.5% vs. 12.7%, p=0.031). On multivariate analysis, the preventive use of pantoprazole (relative hazard: 0.220, 95% CI: 0.051- 0.827, p=0.026) and the specimen size (≥34 mm, relative hazard: 4.178, 95% CI: 1.229-14.197, p=0.022) were two independent factors predictive of delayed bleeding. There were no significant differences in en bloc and complete resection rate between the two groups. In conclusion, pantoprazole is more effective than famotidine for the prevention of delayed bleeding after ESD.
Endoscopic mucosal resection (EMR) is emerging as one of the most important techniques to be incorporated into gastrointestinal endoscopy (1). However, en bloc resection is often not achieved using conventional techniques such as the strip biopsy or cap EMR (2). In an attempt to overcome this limitation, endoscopic submucosal dissection (ESD) techniques using a variety of knives, such as the insulated-tip or the triangle-tip models, have been developed (3-5). ESD can completely remove affected mucosa by dissecting through the middle or deeper part of the submucosa (6). The large specimen extending into the submucosa attained by submucosal dissection techniques permits definitive pathological assessment of resection margins and invasion depth even for large tumors greater than 20 mm in diameter. This would almost certainly result in a lower recurrence rate than piecemeal resection (1).
ESD can achieve a complete resection in a majority of patients, but it is associated with a higher risk of bleeding than classic EMR (7). Although bleeding during ESD is usually treated with procedures such as electrocoagulation and hemoclipping and poses no clinical problem, postoperative or delayed bleeding, on the other hand, has been reported otherwise (8). Prevention of bleeding after ESD is therefore an important clinical issue. For preventing bleeding after ESD, histamine2-receptor antagonists (H2RAs) or proton pump inhibitors (PPIs) have been administered. However, few studies have been made to investigate the effectiveness of H2RAs or PPIs for preventing bleeding after ESD. Therefore, the aim of this study was to compare the relative effectiveness of pantoprazole and famotidine for the prevention of delayed bleeding after ESD.
We conducted a prospective, randomized, single-blind comparative trial. All patients undergoing ESD for gastric neoplasm between September 2005 and September 2006 were included in this prospective study. They were randomly assigned, before gastric ESD, to pantoprazole or famotidine medication. In the pantoprazole group, pantoprazole 80 mg (a loading dose) was given intravenously 2 hr before ESD, and 8 mg/hr of intravenous pantoprazole was given continuously for the first 24 hr (maintenance). At the second day, pantoprazole 40 mg was given intravenously twice. From the third day after ESD, pantoprazole 40 mg was administered orally for 8 weeks. In the famotidine group, famotidine 20 mg was given intravenously twice daily for 2 days, starting 2 hr before ESD. From the third day after ESD, famotidine 20 mg was administered orally twice daily for 8 weeks. The indications for ESD were followings: gastric adenoma (no limitation in size) and an early gastric adenocarcinoma (well or moderately differentiated; size <2 cm if elevated, <1 cm if depressed; no ulceration; and no lymph node involvement or metastasis by CT) (6). Patients were excluded if they had a previous history of upper gastrointestinal surgery or vagotomy; known hypersensitivity to pantoprazole or famotidine; current use of aspirin, non-steroidal anti-inflammatory drugs, or corticosteroids. Possible complications of ESD were discussed with the patients and their relatives, and written informed consent was obtained before entry into the trial. The Ethics Committee of Chonnam National University Hospital approved the treatment protocol. The allocation of patients to treatment was done by drawing sequentially numbered envelopes, each containing a previously determined, randomly selected assignment based on a table of random numbers.
Demographic and clinical characteristics, including age and gender, were recorded. The location, size, and histopathological type of lesions were recorded before ESD. Patients were evaluated for Helicobacter pylori infection by rapid urease test and endoscopic biopsy specimens. Biopsy specimens were stained with H&E and Giemsa. The presence of H. pylori infection was considered negative only if rapid urease test and biopsy specimen were both negative.
ESD was performed by endoscopic submucosal dissection method using an insulated-tip diathermic knife for en bloc resection. After the procedure, the size of tissue specimen, any immediate complications, and histopathologic findings were recorded. ESD was considered complete when the neoplastic tissue was circumferentially surrounded by mucosal and submucosal non-neoplastic tissue (2). ESD was considered to be incomplete when neoplastic tissue was present at the mucosal and/or submucosal margins of the ESD specimen and no additional resections at the periphery of tumor had been taken that showed tumor-free margins on histologic examination (2). Bleeding encountered during ESD was termed immediate, and bleeding after ESD was termed delayed (8). After ESD, patients were observed for vital signs and complete blood counts. Delayed bleeding was suspected as one or more of the ongoing bleeding signs including fresh hematemesis, hematochezia, instability of vital signs, or a reduction of globin by more than 2 g/dL after ESD. When delayed bleeding was suspected, immediate endoscopy was performed. Endoscopic findings determined to indicate bleeding after ESD were 1) active bleeding during re-endoscopy; 2) exposed vessels and/or fresh clots that were not seen immediately after initial ESD; and 3) evident increase in clots in stomach at the re-endoscopy compared with ESD (10). If bleeding or visible vessel was found during ESD and re-endoscopy, thermal coagulation or endoscopic hemoclipping was performed as required. The delayed bleeding rate as a primary outcome was compared between two groups, and immediate bleeding rate, en bloc resection rate, complete resection rate, need for surgery, and mortality, as secondary outcomes, were also compared between two groups. Follow-up endoscopy was performed at 1 month and 3 months after ESD to check the healing process of the ulcer.
Continuous data were summarized as mean (95% confidence interval [CI]). The Student t-test was utilized to compare the mean values of continuous variables. The chi-square test with Yates' correction for continuity and the Fisher's exact test were utilized as appropriate for the comparison of categorical variables. Univariate and forward stepwise multivariate logistic regression analysis were performed to assess the potential predicting factors of delayed bleeding after ESD. The analysis was performed with statistical software package (SPSS 12.0 version for Windows, SPSS, Chicago, IL, U.S.A.). A p value less than 0.05 was accepted as statistically significant. This study hypothesized a reduction of delayed bleeding rate from 20% to 5% by acid suppression therapy. According to the sample size calculation, the study would require 76 patients in each group. The type I error and type II error were set to 0.05 and 0.2, respectively.
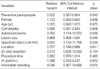
During the study period, a total of 176 patients were enrolled and randomly assigned equally to the two groups. Among them, 8 patients (2 in pantoprazole group and 6 in famotidine group) were excluded from the final analyses because they were lost to follow-up. Four patients (1 in the pantoprazole group and 3 in the famotidine group) were excluded because of a failure to meet the requirements of the final analyses. The cause of exclusion in the pantoprazole group was no loading dose. The cause of exclusion in the famotidine group was current medication of PPIs. Finally, 85 patients in the pantoprazole group and 79 patients in the famotidine group were analyzed. Data regarding the clinical and endoscopic features of the patients are outlined in Table 1. There were no significant differences between two groups with respect to age, gender, comorbidity, histology, location of lesion, positive H. pylori, lesion size, specimen size, and procedure time.
A summary of clinical outcome data is outlined in Table 2. There was no significant difference in the immediate bleeding rate during ESD between the two groups (62.4% vs. 57.0%, p>0.05). However, the delayed bleeding rate after ESD was significantly lower in the pantoprazole group than in the famotidine group (3.5% vs. 12.7%, p=0.031). Immediate bleeding and delayed bleeding were controlled and stopped by electrocoagulation and/or hemoclipping. None of the patients indicated major bleeding requiring surgery. Most of delayed bleeding occurred within 7 days after ESD (11/13, 84.6%). The use of pantoprazole was very effective in preventing bleeding within 24 hr after ESD (0% vs. 6.3%, p= 0.024). Active bleeding (spurting or oozing) was noted in 7/13 (53.8%) of the patients. There were no differences in the en bloc resection rate, complete resection rate, perforation rate, necessity of surgery, and mortality between two groups. Delayed gastric perforation was found in one famotidine-treated patient on the third day after ESD, and he was promptly treated by surgery. Two patients in each group underwent surgery due to vertical margin-positive adenocarcinoma in the resection specimen. During follow-up, no significant side effects induced by drug administration were found in either treatment groups.
Univariate analysis (Table 3) showed that the delayed bleeding was significantly related to the use of preventive pantoprazole (relative hazard: 0.252, p=0.042), adenocarcinoma (relative hazard: 3.765, p=0.026), and specimen size (≥34 mm, relative hazard: 3.652, p=0.030). On multivariate analysis, the use of preventive pantoprazole (relative hazard: 0.220, 95% CI: 0.051-0.827, p=0.026) and specimen size (≥34 mm, relative hazard: 4.178, 95% CI: 1.229-14.197, p=0.022) were two independent predicting factors of delayed bleeding (Table 4).
EMR shows a promising therapeutic option for the removal of early-stage gastric cancer because it is less invasive for patients compared with surgery. To accomplish ultimate cure by EMR, en bloc resection is critical for gastric neoplasm because it may permit accurate histologic diagnosis, definitive pathological assessment of resection margins and invasion depth, and prediction of presence of lymph node metastasis. ESD is a new EMR technique, which has major advantages in comparison with conventional EMR; first, no limitations in location and shape of the lesion; second, en bloc resection is possible even in a large tumor; and third, the tumors with ulcer also are resectable (9). Although ESD can increase en bloc and complete resection rate and may reduce the local recurrence rate, ESD has been reported to have a higher bleeding rate than conventional EMR (7). In ESD, the rate of bleeding after the procedure was reported to be 7% to 38% (3, 10-13). A larger and deeper artificial ulceration by ESD is believed to increase the risk of bleeding in comparison with classic EMR.
In general, PPIs or H2RAs are used for inducing rapid healing of artificial gastric ulcers after EMR. An intragastric pH of greater than 6 has been shown to lower the risk of recurrent bleeding in patients with bleeding peptic ulcers (14). An intravenous bolus followed by continuous-infusion PPI is effective in decreasing rebleeding in patients who have undergone successful endoscopic therapy (15, 16). PPIs are known to be more potent at elevating the intragastric pH than H2RAs. Therefore, it can be assumed that PPIs in duce ulcer healing more rapidly and prevent bleeding episodes more efficiently than H2RAs after ESD. However, to date there have been no studies indicating the effectiveness of PPIs or H2RAs for preventing bleeding after ESD. In a recent study, no difference was found between famotidine and omeprazole in bleeding rates after EMR (17). In that study, bleeding after EMR was seen in five (17.9%) of the 28 patients treated with famotidine and in four (13.8%) of the 29 patients treated with omeprazole (17). However, in the present study, the delayed bleeding rate after ESD was significantly lower in the pantoprazole group than in the famotidine group (3.5% vs. 12.7%, p<0.05). There are several possible explanations for these differences in the delayed bleeding rate. First, the sample size in the previous study was relatively small (28 patients in the famotidine group and 29 patients in the omeprazole group). Second, this may be because of the difference of PPI regimen. The dose of omeprazole (20 mg, I.V., twice a day) was only 40 mg for the first day of EMR in the previous study, but the dose of pantoprazole (initial 80 mg bolus injection, followed by 8 mg/hr continuous infusion) was 280 mg for the first day of ESD in the present study. It has been already known that the median pH of 6.3 could be achieved by 80 mg+8 mg/hr pantoprazole infusion (18). Therefore, 80 mg+8 mg/hr pantoprazole infusion is more useful for preventing bleeding through clot stabilization at an elevated gastric pH. And, a single oral dose of pantoprazole, 40 mg, resulted in significantly more inhibition of gastric acid secretion than omeprazole, 20 mg, over a 24-hr period (19).
In the present study, 80 mg+8 mg/hr pantoprazole infusion was used only in the first day of ESD, and then 40 mg pantoprazole was used intravenously twice in the second day. In patients after EMR, the risk of delayed bleeding is highest mainly within 24 hr after EMR (20). Therefore, the assumption of the current study was that 80 mg+8 mg/hr pantoprazole infusion in the first day of ESD would have the most important clinical significance for preventing bleeding after ESD. In this study, the use of 80 mg+8 mg/hr pantoprazole infusion is very effective in preventing bleeding within 24 hr after ESD (0% vs. 6.3%, p=0.024).
In the present study, the delayed bleeding rate within 24 hr after ESD was significantly lower in the pantoprazole group than in the famotidine group. Several factors, including location, lesion size, specimen size, EMR method, histologic type of gastric tumors, and presence of immediate bleeding, have been implicated in bleeding by EMR (20-22). The distribution of these factors was similar in both groups in the present study. ESD was performed only by endoscopic submucosal dissection method using an insulated-tip diathermic knife in both groups. Therefore, the difference in the delayed bleeding rate within 24 hr after ESD is unlikely to have been influenced by these factors.
In the present study, the use of preventive pantoprazole (relative hazard: 0.220) and specimen size (≥34 mm, relative hazard: 4.178) were two independent predicting factors of delayed bleeding (Table 4). A larger and deeper artificial ulceration by ESD was easy to bleed because bleeding incidence may depend on the size of the specimen (23). And, a recent study demonstrated that PPIs may be superior to H2RAs in terms of converting active stage ulcers into the healing stage, especially in case with a large iatrogenic ulcers after ESD (24). The present study also suggests that PPIs may be more effective than H2RAs for preventing bleeding after ESD by inducing rapid healing of large artificial ulcers. However, in the present study, high-dose PPI was compared with usual-dose famotidine. Therefore, larger clinical tirals comparing high-dose PPI and high-dose famotidine (or usual-dose PPI and usual-dose famotidine) should be performed to find the best effective method for preventing delayed bleeding after ESD.
In the present study, there was no significant drug interaction in either groups. Extensive analyses have demonstrated that famotidine has no clinically significant interactions with theophylline, diazepam, or phenytoin (25). These results are consistent with famotidine's very limited ability to alter the activity of cytochrome P450 isoenzymes (25). Pantoprazole did not interact with digoxin, nifedipine, theophylline, diazepam, warfarin, phenytoin, or oral contraceptives (26). Pantoprazole has been reported to have few clinical significant drug-drug interactions (26).
In conclusion, pantoprazole is more effective than famotidine for the prevention of delayed bleeding after ESD.
References
1. Lightdale CJ. Endoscopy mucosal resection: This is our turf. Endoscopy. 2004. 36:808–810.
2. Rosch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004. 36:788–801.

3. Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H, Yoshida S. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002. 55:576–581.

4. Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, Sugano K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003. 35:690–694.

5. Miyashita M, Tajiri T, Maruyama H, Makino H, Nomura T, Sasajima K, Yamashita K. Endoscopic mucosal resection scissors for the treatment of early gastric cancer. Endoscopy. 2003. 35:611–612.

6. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003. 57:567–579.

7. Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006. 64:877–883.

8. Okano A, Hajiro K, Takakuwa H, Nishio A, Matsushita M. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc. 2003. 57:687–690.

9. Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi , Schimizu Y, Oka M, Ichinose M, Omata M. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006. 63:243–249.

10. Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001. 33:221–226.

11. Hirasaki S, Tanimizu M, Moriwaki T, Hyodo I, Shinji T, Koide N, Shiratori Y. Efficacy of clinical pathway for the management of mucosal gastric carcinoma treated with endoscopic submucosal dissection using an insulation-tip diathermic knife. Intern Med. 2004. 43:1120–1125.
12. Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002. 56:507–512.

13. Yamamoto H, Yube T, Isoda N, Sato Y, Seikine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999. 50:251–256.

14. Green FW Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. Gastroenterology. 1978. 74:38–43.

15. Lin HJ, Lo WC, Lee FY, Perng CL, Tseng GY. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med. 1998. 158:54–58.

16. Lau JY, Sung JJ, Lee KK, Yung MY, Wong SK, Wu JC, Chan FK, Ng EK, You JH, Lee CW, Chan AC, Chung SC. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding ulcers. N Engl J Med. 2000. 343:310–316.
17. Yamaguchi Y, Katsumi N, Tauchi M, Toki M, Nakamura K, Aoki K, Morita Y, Miura M, Morozumi K, Ishida H, Takahashi S. A prospective randomized trial of either famotidine or omeprazole for the prevention of bleeding after endoscopic mucosal resection and the healing of endoscopic mucosal resection-induced ulceration. Aliment Pharmacol Ther. 2005. 21:Suppl 2. 111–115.

18. van Rensburg CJ, Hartmann M, Thorpe A, Venter L, Theron I, Luhmann R, Wurst W. Intragastric pH during continuous infusion with pantoprazole in patients with bleeding peptic ulcer. Am J Gastroenterol. 2003. 98:2635–2641.

19. Pratha V, Hogan DL, Lane JR, Williams PJ, Burton MS, Lynn RB, Karlstadt RG. Inhibition of pentagastrin-stimulated gastric acid secretion by pantoprazole and omeprazole in healthy adults. Dig Dis Sci. 2006. 51:123–131.

20. Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002. 55:390–396.

21. Nelson DB, Block KP, Bosco JJ, Burdick JS, Curtis WD, Faigel DO, Greenwald DA, Kelsey PB, Rajan E, Slivka A, Smith P, Wassef W, Vandam J, Wang KK. Endoscopic mucosal resection: May 2000. Gastrointest Endosc. 2000. 52:860–863.
22. Shiba M, Higuchi K, Kadouchi K, Montani A, Yamamori K, Okazaki H, Taguchi M, Wada T, Itani A, Watanabe T, Tominaga K, Fujiwara Y, Hayashi T. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005. 11:7335–7339.

23. Inoue H, Tani M, Nagai K, Kawano T, Takeshita K, Endo M, Iwai T. Treatment of esophageal and gastric tumors. Endoscopy. 1999. 31:47–55.

24. Ye BD, Cheon JH, Choi KD, Kim SG, Kim JS, Jung HC, Song IS. Omeprazole may be superior to famotidine in the management of iatrogenic ulcer after endoscopic mucosal resection: a prospective randomized controlled trial. Aliment Pharmacol Ther. 2006. 24:837–843.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download