Abstract
Toll-like receptors (TLRs) are pattern-recognition receptors that are important in innate immune responses to bacterial infection. The purpose of this study is to describe the prevalence of TLRs genetic variations in the bacteremic patients in Korea. A total of 154 patients with bacteremia and 179 healthy volunteers were included. The Asp299Gly and Thr399Ile allele of the TLR4 gene and Arg753Gln and Arg677Trp allele of the TLR2 gene were tested by PCR-RFLP. The DNA sequences were determined to confirm the PCR-RFLP results. Contrary to the expectation, no genetic polymorphisms were detected in both groups of this study, suggesting that it is very rare in Korean.
The essential function of the innate immune system stems from its ability to provide a rapid response, acting directly on the pathogen without the need for induction or maturation. The role of Toll signaling in innate immunity in flies was initially studied in the setting of antifungal responses to the pathogen Aspergillus fumigatus (1). Toll-like receptors (TLRs), with the exception of TLR9 which exists in the cytoplasm, are all transmembrane molecules. The extracellular amino termini have variable leucine-rich repeat domains, which are involved in the recognition of pathogen-associated molecular patterns (PAMPs): TLR2 for peptidoglycans (2, 3), lipoteichoic acid (2), lipoarabinomannan (3), and bacterial lipoproteins (4); double-stranded RNA by TLR3; TLR4 for lipopolysaccharide (LPS) (5), and heat-shock protein (hsp); TLR5 for flagellin (6); TLR9 for bacterial DNA (7), and the intracellular domains contain a conserved Toll/interleukin-1 (IL-1) receptor (TIR) domain (8). To date, a total of 10 human TLRs have been identified (9), and TLR11 is being studied in mice and protozoa (9, 10).
Arbour et al. demonstrated 2 cosegregating missense mutations in the extracellular domain of the receptor of the human TLR4 (hTLR4) gene, an aspartic acid-to-glycine substitution at position 299 of the amino acid sequence (Asp299Gly) and a threonine-to-isoleucine substitution at position 399 of the amino acid sequence (Thr399Ile). These mutations are known to be associated with hyporesponsiveness to LPS and an increased incidence of Gram-negative septic shock, which was initially demonstrated by the observation that LPS-hyporesponsive C3H/HeJ mice have a point mutation in the TLR4 gene (11, 12). TLR2 is found in substantial amounts on monocytes and neutrophils, but also on dendritic cells. Studies employing TLR2 knock out mice revealed a higher susceptibility to infections caused by Gram-positive bacteria, spirochetes, and mycobacteria (13-15).
We focused on TLR4 (Asp299Gly, Thr399Ile) and TLR2 (Arg753Gln, Arg677Trp) mutations, because they have been the most extensively studied and are known to play prominent roles in response to Gram-negative and Gram-positive infections (16). The purpose of this study is to describe the prevalence of these genetic variations in the bacteremia patients and in healthy volunteers in Korea.
A total of 154 patients with bacteremia and 179 healthy volunteers at Yongdong Severance Hospital were recruited between March 2003 and September 2004. Patients who had at least one positive blood culture and took antibiotics for treatment were included in this study. The informed consent was received from all patients and volunteers.
Whole blood was collected from the study subjects, and genomic DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's protocol, including RNase-free DNase digestion (17). PCR primers were designed to allow a distinction of wild-type and mutant TLR4 alleles based on the presence of restriction enzyme recognition sites (17). In both cases, the forward primer sequences were altered to generate a restriction site in the mutant allele. For TLR2, we designed primers spanning a region of 340 bp including both polymorphisms (Arg753Gln, Arg677Trp) (27) (Table 1). Reactions were set up using the AmpliTaq® PCR kit (Applied Biosystems, Foster City, CA, U.S.A.). In a total reaction volume of 50 µL, 5 µL 10×PCR buffer, 20 pM each primer, 0.1 µg genomic DNA, 2.5 U Taq DNA polymerase, 200 µM dNTP mixture, and 1.5 mM magnesium chloride were combined. Reactions were run on a GeneAmp PCR System 2400 (Applied Biosystems) using the following conditions: 94℃ for 5 min, then 30 cycles of 94℃ for 30 sec, 55℃ (58℃ for TLR2) for 30 sec, and 72℃ for 30 sec. QIAquick Gel Extaraction Kit (Qiagen, Hilden, Germany) was used for extracting and purifying amplicons after PCR. Five microliters of the resulting products were used for an overnight digest with the appropriate restriction enzyme (Table 1) in a total volume of 20 µL at 37℃, and digests were run out on a 4% MetaPhore® agarose gel (BMA, Rockland, ME, U.S.A.) to determine the TLR2 and TLR4 alleles. To confirm the mutant TLR2 and 4 alleles, the DNA sequences were determined with ABI Prism® 3100 Genetic Analyzer (Applied Biosystems, CA, U.S.A.) in all PCR products
The mean age of the bacteremia patients was 58.7±17.2 yr, and the sex ratio was 84 men to 70 women. The mean age of the volunteers was 47.0±14.0 yr, and the sex ratio was 102 men to 77 women. Specific organisms were identified in bacteremia patients.
Primary sites of bacteremia in patient group were as follows: urine 31 (20.1%), wound 18 (11.7%), lung 9 (5.8%), catheter 7 (4.5%), abscess 5 (3.3%), bile 3 (1.9%), peritoneal fluid 2 (1.9%), throat 2 (1.9%), others 5 (3.3%), and unknown sites 72 (46.8%). In the bacteremia patients, there were 79 (51.3%) Gram-positive infections, 65 (42.2%) Gram-negative infections, 2 (1.3%) anaerobic infections and 8 (5.2%) fungal infections. Staphylococcal infections were 60 (75.9%) out of 79 Gram-positive infections. Compared to the positive control of PCR-RFLP pattern of Asp299Gly (223 bp), which has a restriction site by NcoI, the band from all specimens of this study appeared on 249 bp which represents for a wild type. So there were no Asp299Gly polymorphisms in any of the specimens. If another TLR4 polymorphism, Thr399Ile, was present, it should be restricted by HinfI and the band should be noted on 378 bp. However, the band appeared on 410 bp which represents for a wild type, too.
As for the TLR2 wild type, there are two restriction sites by AciI. From these restriction sites, three bands appear on 38 bp, 75 bp, and 227 bp in wild type. If TLR2 polymorphisms (Arg753Gln or Arg677Trp) are present, there is only one restriction site and therefore, only two bands (75 bp, 265 bp in Arg753Gln and 38 bp, 302 bp in Arg677Trp) should be appeared. In our results, however, three bands appeared in all the patients and healthy volunteers, which represented for wild type (Fig. 1). To verify these negative findings, we performed sequencing analysis on all samples, and we confirmed that there were no polymorphisms in this study subjects.
Susceptibility to lethal infections throughout a person's lifetime may be significantly dependent on genetic factors such as genetic polymorphisms (16, 18). The role of a TLR4 polymorphism on the susceptibility to infections is still controversial and it is currently unresolved whether a hyporesponsive LPS signaling pathway is beneficial or detrimental to the host (11, 19-21).
The frequency of the Asp299Gly allele of the TLR4 gene has been reporting as variable range (3-18%) according to the study subjects (19-24). However, Okayama et al. reported that the Asp299Gly allele was not detected in any of the specimens, suggesting that it is very rare in Japanese population (25). Arg753Gln mutation has been found in 2.7% in whites by Lorenz et al. (26), while higher mutation ratio (14.6%) was reported in German (27). While no individuals carrying the Arg677Trp SNP (single nucleotide polymorphism) were identified in a large group of whites (27), it was found in 10 of 45 lepromatous leprosy patients (22%) (28). Kang et al. reported high frequency (22%) of TLR2 mutation in Korean lepromatous leprosy patients (28), but no mutations were identified in controls and tuberculoid leprosy patients. However, our data showed that no polymorphisms were present in bacteremia patients, which is contradictory to the previous studies. It could not be compared directly to the Kang's study (28) because no leprosy patients were included in our study. But the results that there was no mutation in healthy control groups in both studies are similar. Considering variable results as described above, it may suggest that the genetic polymorphisms vary according to the race, organisms, or other certain conditions. Also the genetic polymorphisms may not be functional or TLR function is not rate limiting step in certain organisms or conditions (11, 29). Or other member of the TLR family or their cofactors contribute to effective innate immunity in Korean, even if TLR2 or 4 function or expression is impaired. Compare to the results by Lorenz et al. (26), no Arg753Gln polymorphism was found among 60 staphylococcal infections in this study. This lack of correlation may be due to the rarity of this polymorphism in Koreans or additional genes like TNF-α may be involved in determining the susceptibility to septic shock for Gram-positive organisms (30).
The limitations of our study are as follows. First, we could get only one positive control sample for Asp299Gly and not for others. So we did the sequencing analyses for these loci to confirm our PCR results. Second, we did not examine the full sequence of TLR2 and TLR4, but we only sequenced for the specific gene loci that polymorphism is known to be occurred most frequently. The possibility cannot be ruled out that as-yet-unidentified other polymorphic loci which can influence susceptibility to Gram-negative or -positive infection may be present within TLR4 or 2. Third, our sample size is relatively small, so further increase of sample size of the cohort will be needed.
In spite of these limitations, this report is important as the first research about frequency and correlation of TLR2 and TLR4 polymorphisms to bacteremia in an Asian country. Though our results were negative, additional polymorphisms might be associated with molecular pathogenesis of sepsis. If the sepsis-specific genetic polymorphisms are found from further studies, it will be possible to provide individualized preventive and therapeutic measures for this devastating disease in the future. However, larger sized studies are needed.
Figures and Tables
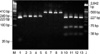
Fig. 1
Representative results of samples tested for TLR 2 and 4 polymorphisms with the PCR-RFLP.
M: DNA 100 bp ladder size marker; 1: TLR4 (Arg299Gly) positive control (223 bp); 2-3: TLR4 (299) in bacteremia (249 bp); 4-5: TLR4 (299) in healthy control (249 bp); 6-7: TLR4 (399) in bacteremia (410 bp); 8-9: TLR4 (399) in healthy control (410 bp); 10-11: TLR2 in bacteremia (3 bands: 38 bp, 75 bp, 227 bp); 12-13: TLR2 in healthy control (3 bands: 38 bp, 75 bp, 227 bp); λ: DNA 50 bp ladder size marker.
References
1. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996. 86:973–983.
2. Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999. 274:17406–17409.

3. Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999. 11:443–451.

4. Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrellia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J Immunol. 1999. 163:2382–2386.
5. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998. 282:2085–2088.

6. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001. 410:1099–1103.

7. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K. A Toll-like receptor recognizes bacterial DNA. Nature. 2000. 408:740–745.

8. Vasselon T, Detmers PA. Toll receptors: a central element in innate immune responses. Infect Immun. 2002. 70:1033–1041.

9. Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghost S. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004. 303:1522–1526.

10. Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005. 308:1626–1629.

11. Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000. 25:187–191.

12. Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002. 162:1028–1032.

13. Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient an MyD88-deficient mice are highly susceptible to Staphylococcus infection. J Immunol. 2000. 165:5392–5396.
14. Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002. 186:798–806.
15. Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdoferi. J Immunol. 2002. 168:348–355.
17. Lorenz E, Frees KL, Schwartz DA. Determination of the TLR4 genotype using allele-specific PCR. Biotechniques. 2001. 31:22–24.

18. Angus DC, Burgner D, Wunderink R, Mira JP, Gerlach H, Wiedermann CJ, Vincent JL. The PIRO concept: P is for predisposition. Crit Care. 2003. 7:248–251.
19. Agnese DM, Calvano JE, Hahm SJ, Coyle SM, Corbett SA, Calvano SE, Lowry SF. Human Toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002. 186:1522–1525.

20. Morre SA, Murillo LS, Bruggeman CA, Pena AS. The role that the functional Asp299Gly polymorphism in the Toll-like receptor 4 gene plays in susceptibility to Chlamydia trachomatis-associated tubal infertility. J Infect Dis. 2003. 187:341–342.
21. Svanborg C, Fréndeus B, Godaly G, Hang L, Hedlund M, Wachtler C. Toll-like receptor signaling and chemokine receptor expression influence the severity of urinary tract infection. J Infect Dis. 2001. 183:Suppl 1. S61–S65.

22. Read RC, Pullin J, Gregory S, Borrow R, Kaczmarski EB, di Giovine FS, Dower SK, Cannings C, Wilson AG. A functional polymorphism of Toll-like receptor 4 is not associated with likelihood or severity of meningococcal disease. J Infect Dis. 2002. 184:640–642.

23. Feterowski C, Emanuilidis K, Miethke T, Gerauer K, Rump M, Ulm K, Holzmann B, Weighardt H. Effects of functional Toll-like receptor 4 mutations on the immune response to human and experimental sepsis. Immunology. 2003. 109:426–431.
24. Morre SA, Murillo LS, Spaargaren J, Fennema HS, Pena AS. Role of Toll-like receptor 4 Asp299Gly polymorphism in susceptibility to Candida albicans infection. J Infect Dis. 2002. 186:1377–1379.
25. Okayama N, Fujimura K, Suehiro Y, Hamanaka Y, Fujiwara M, Matsubara T, Maekawa T, Hazama S, Oka M, Nohara H, Kayano K, Okita K, Hinoda Y. Simple genotype analysis of the Asp299Gly polymorphism of the Toll-like receptor-4 gene that is associated with lipopolysaccharide hyporesponsiveness. J Clin Lab Anal. 2002. 16:56–58.
26. Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000. 68:6398–6401.

27. Schröder NW, Hermann C, Hamann L, Gobel UB, Hartung T, Schumann RR. High frequency of polymorphism Arg753Gln of the Toll-like receptor-2 gene detected by a novel allele-specific PCR. J Mol Med. 2003. 81:368–372.

28. Kang TJ, Chae GT. Detection of toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol Med Microbiol. 2001. 31:53–58.

29. Ingalls RR, Lien E, Golenbock DT. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitides activate the inflammatory response through Toll-like receptor 2. Infect Immun. 2001. 69:2230–2236.
30. Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riche F, Leleu G, Arbibe L, Mignon A, Delpech M, Dhainaut JF. Association of TNF2, a TNF-α promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA. 1999. 282:561–568.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download