Abstract
We report on the investigations and interventions conducted to contain an extended outbreak of Serratia marcescens bacteriuria that lasted for years in a neurosurgical intensive care unit (NSICU). A case-control study was performed to identify the risk factors for S. marcescens acquisition in urine. In case patients, urine sampling for tests and central venous catheterization were performed more frequently before the isolation of S. marcescens. Case patients were more frequently prescribed third-generation cephalosporins. Adherence to hand antisepsis was encouraged through in-service educational meetings and infection control measures, especially concerning the manipulation of indwelling urinary catheters, were intensified. The outbreak persisted despite the reinforcement of infection control measures. However, no patient has newly acquired the organism in the NSICU since December 2004. Multiple factors, including inadequate infection control practices and inappropriate antimicrobial usage, possibly contributed to the persistence of this S. marcescens outbreak. Healthcare workers should consistently follow infection control policies to ensure quality care.
Serratia marcescens is an aerobic Gram-negative bacillus which can survive well in moist environments (1). S. marcescens has been reported to cause variable infections, including respiratory tract, urinary tract, and wound infections and bacteremia (1-3). S. marcescens often develops multidrug resistance and tends to spread rapidly in the nosocomial environment (4-9), and has been implicated in outbreaks of nosocomial infection both in neonates and adults. S. marcescens is more likely to colonize the respiratory and urinary tracts of hospitalized adults but the gastrointestinal tract in neonates (1, 10-12).
Numerous outbreaks of S. marcescens in urine have been described (9, 13-23). Only rarely is the source of an outbreak in urine identified, i.e., urodynamic equipment, urinometers, or urine measuring containers (15, 16, 18). In most instances, the presumed source of infection is healthcare workers (1, 7, 10, 13, 17). In our hospital, an increase in the number of cases of S. marcescens was observed in urine over the past few years, with unusual accumulations of isolates in the neurosurgical intensive care unit (NSICU) as of March 2002. The purpose of this study was to investigate this outbreak of S. marcescens bacteriuria, and to report interventions.
Keimyung University Dongsan Medical Center is a 931-bed tertiary care hospital in Daegu, Korea. The NSICU involved in the outbreak is a 20-bed unit with two private rooms, and is an integral part of a single ward consisting of 61 beds devoted to neurosurgical patients. Our Infection Control Unit with a full-time infection control nurse was established in June 2000 but no Infectious Disease service was established until March 2002. This study was conducted as part of a quality improvement project in the NSICU in 2003.
To determine the trends of isolation of S. marcescens over time, we used stored computerized microbiology data dating from July 1997. Microbiologic data were analyzed by culture specimen, hospital ward, and clinical department.
We examined patient care areas and other areas of the NSICU and reviewed infection control policies and hand antisepsis practices in May 2002. Additionally, we surveyed the infection control practices of health care workers, especially in terms of the manipulation of indwelling urinary catheters.
A case-control study was conducted in May 2002 to identify the risk factors for the acquisition of S. marcescens in urine. The source population for this case-control study was defined as all patients who had been admitted to the NSICU for ≥ 72 hr from January through March 2002. A case patient was defined as any patient in the NSICU with at least one positive culture for S. marcescens in urine. Data were collected until the date of isolation of S. marcescens in urine. For each case, two controls were selected from patients without isolation of S. marcescens in urine as close as possible in time to the case. Collection of data ended after a maximum of 7 days in the NSICU, which was the median length of ICU stay before the isolation of S. marcescens in the case patients.
Environmental cultures were obtained from potential environmental sources, such as, sinks, counter tops, bed rails, medication carts, urinary catheters and urine bags, unused urinals, and disinfectants in February 2003. Cultures were not obtained from healthcare workers. Prospective surveillance cultures were obtained from urine for all patients with indwelling urinary catheters in the NSICU. Urine samples were taken on the day of and 3 days after admission to the NSICU, and thereafter weekly. Surveillance cultures were continued weekly in the NS ward as long as urinary catheters were kept in place after the patients were transferred to the NS ward. Routine surveillance cultures were conducted from February through December 2003, but not thereafter due to limited resources. Additional surveillance cultures to measure point prevalences were done during April 2005. Cultivation and identification of the organism were performed according to standard procedures. Pulsed-field gel electrophoresis (PFGE) was performed for 34 S. marcescens strains isolated from April 2002 to November 2003 as described elsewhere (23) and their fragmentation patterns were interpreted using previously suggested criteria (24).
Univariate analysis was performed using the χ2 test or the two-tailed Fisher's exact test (for small expected values) for categorical variables, and using the Student's t-test for continuous variables. Multivariate analysis was performed to demonstrate associations between individual variables and organism acquisition. All variables that were statistically significant by univariate analysis were analyzed using a logistic regression model. Two-tailed p values of <0.05 were considered statistically significant. All statistical analyses were performed using SPSS software (SPSS 10.0 for Windows).
From July 1997 through December 2001, a total of 1,231 S. marcescens duplicate isolates among 525 hospitalized patients were recovered from a variety of clinical specimens. The organisms were most commonly isolated from urine specimens (71.2%) and from the NS (82.0%). Of 389 patients with S. marcescens in urine, 340 (87.4%) were from the NS. For most of these NS patients, acquisition of S. marcescens was identified while they were in the NSICU (81.8%). It was evident that the outbreak was associated with urine, particularly in the NSICU.
The first isolate of S. marcescens from urine in the NSICU was identified in a 51-yr old male patient transferred from other hospital for surgical management of cerebral hemorrhage in July 1999. At the time of admission to the NSICU, indwelling urinary catheter had already been placed at the previous hospital and he revealed no signs of infection. On hospital day 8, the organism was cultured from catheterized urine, and found to be resistant to multiple antibiotics but susceptible to imipenem. Thereafter S. marcescens was increasingly identified in urine of patients in the NSICU and then in the NS ward (Fig. 1). Almost all organisms showed the similar antibiotic susceptibility pattern, although different sets of antibiotics were used for antimicrobial susceptibility testing during the study period. We believe that the above transferred patient was the index case of this outbreak.
The demographic and clinical characteristics of 35 cases and 70 control patients are shown in Table 1. Subarachnoid hemorrhage was the most common diagnosis and no statistical difference was found in the distribution of neurosurgical diseases between cases and controls. Urine sampling for tests and invasive procedures were more frequently performed in case patients. Furthermore case patients received antibiotics for longer periods for surgical prophylaxis or empiric treatment of fever of unknown etiology, and this was especially true for third-generation cephalosporins, such as cefoperazone. Mean length of NSICU stay before the isolation of S. marcescens in urine was 6.9 days (median, 7; range, 3-11 days). All isolates were resistant to multiple antibiotics (including cefoxitin, cefotaxime, cefepime, gentamicin, and amikacin) and only susceptible to imipenem. Only one isolate was susceptible to ciprofloxacin. Multivariate analysis revealed that the use of third-generation cephalosporins, urine sampling for tests, and central venous catheterization were independent risk factors for the acquisition of S. marcescens (Table 2).
Written infection control standards were in place at the hospital, but these were not strictly followed by healthcare workers in the NSICU. Breaks in infection control practices were identified during the manipulation of indwelling urinary catheters. Backflow of urine through a urinary catheter was often found at the time of position changes and during patient movements. Connection tubes were disconnected for urine sampling for urinalysis, culture, or for the measurement of specific gravity. The irrigation of urinary catheters was performed often but not routinely. A few urinals were shared to drain urine collected in urine bags and they were not sufficiently disinfected between usages according to the current infection control standards (25). Furthermore, hand antisepsis between the nursing care of individual patients was inadequate. However, inappropriate practices were not documented at the time of catheter insertion.
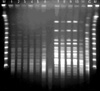
In a patient colonized with S. marcescens in urine, environmental cultures isolated S. marcescens, Staphlyococcus epidermidis, and Acinetobacter baumannii from the surface of a urine bag. Results of surveillance cultures are depicted with those of clinical specimens in Fig. 1. Last point prevalence surveillance cultures in April 2005 did not grow S. marcescens from any patient. PFGE of 34 selected strains isolated between April 2002 and November 2003 identified two distinct clones (Fig. 2). One predominant clone distributed throughout the period above, was comprised of two closely related subtypes, and was resistant to all antibiotics tested, except imipenem. The other clone showed different antimicrobial susceptibility patterns (Table 3). This clone had been isolated since October 2003.
After the procedural review and survey, adherence to hand antisepsis was encouraged and infection control measures, especially on the manipulation of indwelling urinary catheters (26), were intensified through in-service educational meetings for nursing staffs in the NSICU and NS ward in January 2003. Individual urinals were not recommended for economic reasons but it was recommended that drainage valves not be touched when draining urine. A pair of new plastic, not sterile, gloves were used each time urine was drained from a urine bag. Disconnection of connection tubes was not allowed thereafter. Urine sampling was performed only by aspiration with a sterile needle and syringe after clamping the distal end of the catheter and disinfection, because no sampling port was present in available catheters. In addition, it was reinforced against irrigation through indwelling urinary catheters. Cohort care was employed for patients acquiring S. marcescens in urine. For residents and attending medical staffs, a series of lectures was given on the state of the S. marcescens outbreak in the NSICU in May 2003. The outbreak lasted despite the reinforcement of infection control measures, but eventually no patients were found to have newly acquired the organism in the NSICU from December 2004.
This study describes an extended outbreak of S. marcescens in an NSICU that lasted for years. It is hypothesized that the outbreak strain might have been introduced into our NSICU by a neurosurgical patient who was transferred from other hospital with a urinary catheter in place.
Majority of postoperative neurosurgical patients are usually admitted for intensive care with invasive monitoring (27). Indwelling urinary catheters are often placed and regular urine measurements, such as volume and specific gravity, are performed for fluid and electrolyte management. In the case-control study, urine sampling for tests was found to be significantly associated with the acquisition of S. marcescens by multivariate analysis. A procedural investigation revealed poor infection control standards, especially in terms of the management of urinary catheters, which contributed to the establishment of the S. marcescens outbreak in the NSICU. Although the exact number and intensity of direct contacts between patients and healthcare workers were not collected, and cultures were not obtained from the hands of healthcare workers, we presume that after the introduction of the epidemic strain, hand transmission probably played a major role in the dissemination and persistence of S. marcescens. Therefore, careful hand hygiene and strict adherence to basic infection control practices were the key step to terminating this outbreak.
It is noteworthy that, even when there was a high awareness of existing problems and a generally high infection control level, it was exceedingly difficult to contain the outbreak. One possible factor that contributed to its duration was prolonged hospitalization. Some of the neurosurgical patients stayed in the hospital for long periods for medical and surgical reasons. A few of these patients were repeatedly transferred from the NSICU to the ward or vice versa, and a small number of these patients had already acquired the organism in urine before transfer. It is possible that new transmissions from patients who were already colonized, but not identified, occurred after the initial intervention during the period of the outbreak. These patients may have been another cause of the persistence and resurgence of the organism during intervention. Prolonged hospitalization was suggested to be a risk factor of a S. marcescens outbreak in a previous study (8).
Another factor that may have contributed to the outbreak was the use of broad-spectrum antibiotics. Case patients received antibiotics more frequently and for longer periods for surgical prophylaxis and for the empiric treatment of fever of an undefined cause, especially with third-generation cephalosporins, such as cefoperazone. Antibiotic treatment was likely to have predisposed these patients to the acquisition of S. marcescens in the NSICU. It is known that third-generation cephalosporins have the ability to select S. marcescens in the gut (28).
S. marcescens isolates from the NSICU were multidrug resistant and of the antibiotics tested the predominant strain was only susceptible to imipenem. Nosocomial outbreaks of S. marcescens are commonly associated with multiple antimicrobial resistance and extended-spectrum beta-lactamase producing isolates were recently reported from an ICU (4-6, 8). The emergence and persistence of multidrug resistant S. marcescens is clearly a cause of concern because of treatment failure. Since S. marcescens has inducible chromosomal beta-lactamases (29), it is prudent to avoid the long-term use of antibiotics like third-generation cephalosporins, for surgical prophylaxis. Nevertheless, no significant changes in antibiotic policy or prescription pattern were observed during the course of the outbreak (data not shown). Further efforts will be needed to improve antimicrobial use.
The molecular epidemiologic study showed two different clones. One strain, not major, was identified from a small number of isolates tested for PFGE. S. marcescens organisms with the same antimicrobial susceptibility pattern as this clone were isolated rarely at irregular intervals throughout the outbreak. However, we could not presume that these isolates were identical to the minor clone. Furthermore, we were unable to determine whether or when the new strain had been introduced, because the same set of antibiotics was not used in the clinical microbiology laboratory during the outbreak, and not all S. marcescens isolates in the NSICU were collected for PFGE during the course of this study.
This study has the following limitations. First, we did not detect all S. marcescens colonizations or infections because surveillance cultures were performed during the limited period of the outbreak. In addition, we could not obtain cultures from healthcare workers. Second, the relationship between previous colonization and invasive disease was not demonstrated for patients colonized with S. marcescens in urine. During the period of intervention, systemic infections caused by this organism, such as meningitis and bacteremia, often occurred in patients who had already been colonized with S. marcescens in urine. However, the exact incidence of S. marcescens infections was not estimated in the whole period and thus could not be compared between pre- and post-intervention period because routine surveillance had not been performed especially in the early period of this outbreak. Nevertheless, it can be assumed that the economic and clinical burden was substantial during the outbreak.
In summary, we believe that multiple factors, including unsatisfactory hand washing practices, inadequate infection control practices, and inappropriate antimicrobial usage, contributed to the dissemination and persistence of the S. marcescens outbreak in our NSICU. This study underscores the importance of practicing basic infection control measures in the hospital.
Figures and Tables
Fig. 1
Distribution of patients who newly acquired Serratia marcescens in urine from July 1999 through February 2005.
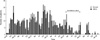
Fig. 2
PFGE fingerprints of Serratia marcescens isolates. One major clone was shared by the isolates shown in lanes 1, 2, 4, and 5 (type A) with 2 closely-related isolates (lanes 3 and 6 designated as subtypes A1 and A2, respectively). Five isolates showed a different pattern (type B, lanes 7-11). Antimicrobial susceptibility patterns of the isolates above are shown according to clone type in Table 3. M, Lambda DNA size marker; C, control (E. coil O157: H7G5244).
References
1. Yu VL. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979. 300:887–893.
3. Wilfert JN, Barrett FF, Kass EH. Bacteremia due to Serratia marcescens. N Engl J Med. 1968. 279:286–289.
4. Sanders CC, Watanakunakorn C. Emergence of resistance to beta-lactams, aminoglycosides, and quinolones during combination therapy for infection due to Serratia marcescens. J Infect Dis. 1986. 153:617–619.
5. Herra CM, Knowles SJ, Kaufmann ME, Mulvihill E, McGrath B, Keane CT. An outbreak of an unusual strain of Serratia marcescens in two Dublin hospitals. J Hosp Infect. 1998. 39:135–141.

6. Pagani L, Luzzaro F, Ronza P, Rossi A, Micheletti P, Porta F, Romero E. Outbreak of extended-spectrum beta-lactamase producing Serratia marcescens in an intensive care unit. FEMS Immunol Med Microbiol. 1994. 10:39–46.
7. Schaberg DR, Alford RH, Anderson R, Farmer JJ III, Melly MA, Schaffner W. An outbreak of nosocomial infection due to multiply resistant Serratia marcescens: evidence of interhospital spread. J Infect Dis. 1976. 134:181–188.

8. Bagattini M, Crispino M, Gentile F, Barretta E, Schiavone D, Boccia MC, Triassi M, Zarrilli R. A nosocomial outbreak of Serratia marcescens producing inducible Amp C-type beta-lactamase enzyme and carrying antimicrobial resistance genes within a class 1 integron. J Hosp Infect. 2004. 56:29–36.

9. Su LH, Ou JT, Leu HS, Chiang PC, Chiu YP, Chia JH, Kuo AJ, Chiu CH, Chu C, Wu TL, Sun CF, Riley TV, Chang BJ. Extended epidemic of nosocomial urinary tract infections caused by Serratia marcescens. J Clin Microbiol. 2003. 41:4726–4732.

10. Farmer JJ III, Davis BR, Hickman FW, Presley DB, Bodey GP, Negut M, Bobo RA. Detection of Serratia outbreaks in hospital. Lancet. 1976. 2:455–459.

11. Newport MT, John JF, Michel YM, Levkoff AH. Endemic Serratia marcescens infection in a neonatal intensive care nursery associated with gastrointestinal colonization. Pediatr Infect Dis. 1985. 4:160–167.

12. Christensen GD, Korones SB, Reed L, Bulley R, McLaughlin B, Bisno AL. Epidemic Serratia marcescens in a neonatal intensive care unit: importance of the gastrointestinal tract as a reservoir. Infect Control. 1982. 3:127–133.

13. Maki DG, Hennekens CG, Phillips CW, Shaw WV, Bennett JV. Nosocomial urinary tract infection with Serratia marcescens: an epidemiologic study. J Infect Dis. 1973. 128:579–587.

14. Madduri SD, Mauriello DA, Smith LG, Seebode JJ. Serratia marcescens and the urologist. J Urol. 1976. 116:613–615.

15. Cann KJ, Johnstone D, Skene AI. An outbreak of Serratia marcescens infection following urodynamic studies. J Hosp Infect. 1987. 9:291–293.

16. Krieger JN, Levy-Zombek E, Scheidt A, Drusin LM. A nosocomial epidemic of antibiotic-resistant Serratia marcescens urinary tract infections. J Urol. 1980. 124:498–502.

17. Okuda T, Endo N, Osada Y, Zen-Yoji H. Outbreak of nosocomial urinary tract infections caused by Serratia marcescens. J Clin Microbiol. 1984. 20:691–695.

18. Rutala WA, Kennedy VA, Loflin HB, Sarubbi FA Jr. Serratia marcescens nosocomial infections of the urinary tract associated with urine measuring containers and urinometers. Am J Med. 1981. 70:659–663.

19. Echols RM, Palmer DL, King RM, Long GW. Multidrug-resistant Serratia marcescens bacteriuria related to urologic instrumentation. South Med J. 1984. 77:173–177.

20. Arroyo JC, Milligan WL, Postic B, Northey J, Parker E, Bryan CS. Clinical, epidemiologic and microbiologic features of a persistent outbreak of amikacin-resistant Serratia marcescens. Infect Control. 1981. 2:367–372.

21. John JF Jr, McNeill WF. Characteristics of Serratia marcescens containing a plasmid coding for gentamicin resistance in nosocomial infections. J Infect Dis. 1981. 143:810–817.

22. Simor AE, Ramage L, Wilcox L, Bull SB, Bialkowska-Hobrzanska H. Molecular and epidemiologic study of multiresistant Serratia marcescens infections in a spinal cord injury rehabilitation unit. Infect Control. 1988. 9:20–27.

23. Koh EH, Kim S, Bae IG. Epidemiological investigation of an outbreak of Serratia marcescens urinary track infection in an intensive care unit using pulsed-field gel electrophoresis. Korean J Clin Microbiol. 2005. 8:34–40.
24. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995. 33:2233–2239.

25. Rutala WA. APIC guideline for selection and use of disinfectants. 1994, 1995, and 1996 APIC Guidelines Committee. Association for Professionals in Infection Control and Epidemiology, Inc. Am J Infect Control. 1996. 24:313–342.
26. Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983. 11:28–36.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download