Abstract
Heart rate (HR) recovery after exercise is a function of vagal reactivation, and its impairment is a predictor of overall mortality and adverse cardiovascular events. While metabolic syndrome is associated with sympathetic overactivity, little is known about the relationship between metabolic syndrome and HR recovery. A symptom-limited exercise stress test in healthy subjects (n=1,434) was used to evaluate HR recovery. Metabolic syndrome was defined according to the National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATP-III) criteria. Seventeen percent of subjects had ≥3 criteria for metabolic syndrome. HR recovery was lower in men than women and in smokers than nonsmokers. The subject with metabolic syndrome (vs. without) showed lower HR recovery (10.3±11.6 vs. 13.6±9.7 per minute) and higher resting HR (64.3±10.3 vs. 61.6±9.1 per minute). HR recovery correlated inversely to age (r=-0.25, p<0.0001), but not to resting HR or maximal oxygen uptake. Delayed HR recovery was associated with metabolic syndrome after an adjustment for age, sex, resting HR and smoking (p<0.01). Metabolic syndrome is associated with impaired vagal reactivation. Adverse cardiovascular outcomes associated with metabolic syndrome may be mediated by the failure of vagal reactivation in addition to sympathetic overactivity.
Delayed heart rate (HR) recovery after exercise is a function of vagal reactivation, and it is a predictor of overall mortality (1,2) and adverse cardiovascular events (3) in the general population who has no prior evidence of clinical cardiovascular disease (CVD). Its predictive value for adverse events has also been shown in patients with ischemic heart disease (4-6), or with diabetes (7). Parasympathetic tone dominates the resting state, while exercise arouses sympathetic activation with the withdrawal of vagal tone. After exercise, HR recovers to the level of normal by parasympathetic activation followed by sympathetic withdrawal (8,9). Therefore, a delayed HR recovery after exercise implies a dysfunction of vagal reactivation. Sympathetic overactivity is known to be associated with hyperinsulinemia or insulin resistance, which underlies the metabolic syndrome (10,11). There have been studies indicating that autonomic dysfunction, involving both sympathetic and parasympathetic activity, is associated with obesity (12), insulin resistance (13), type 2 diabetes (14), and brain hemorrhage (15). Attenuated parasympathetic activity has recently been seen in the first-degree relatives or offspring of type 2 diabetes patients (16,17). However, less is known about the relationship between metabolic syndrome and impaired vagal tone.
Therefore, the aim of this study is to investigate the association between metabolic syndrome and HR recovery, which reflects post-exercise parasympathetic reactivation.
The subjects for the study (n=1,434, M:F=978:456, mean age 51±9 yr) were volunteers for a routine health check-up in the health promotion center, Samsung Medical Center, Seoul, Korea. The subject's past medical histories were obtained through a structured questionnaire. Those who had history of stroke, established heart disease, and/or known diabetes or who were on medication were excluded from the analysis. Twelve percent of the subjects were either hypertensive or on antihypertensive medication and were included in the study. Written consents were obtained from all subjects and the institutional review board approved our study.
Blood sampling for serum lipid profile and glucose was obtained after at least 14 hr of fasting. Metabolic syndrome was defined according to the Adult Treatment Panel III (ATP-III) criteria (18), except that body mass index (BMI) (>25 kg/m2) was substituted for waist circumference (19). The subjects were diagnosed as having metabolic syndrome if 3 or more of the 5 criteria were met; 1) BMI >25 kg/m2, 2) triglycerides ≥150 mg/dL, 3) HDL cholesterol <40 mg/dL in men, <50 mg/dL in women, 4) blood pressure ≥130/≥85 mmHg, 5) fasting blood glucose ≥110 mg/dL.
A maximal exercise stress test was done in all subjects using the modified Bruce protocol. Blood pressure, HR and the Borg scale of rating of perceived exertion (RPE) (20) were measured at 2 min of each stages of the exercise. Exercise was stopped when the subject demanded cessation of the treadmill due to exhaustion, or if the heart rate achieved was more than 90% of estimated maximal HR (220-age), or if the RPE was more than 17 or the respiratory exchange ratio was more than 1.15. During the recovery phase, the subjects continued to walk for 30 sec at the speed of 1.2 mph and then they sat down for 5 min with continued medical monitoring. HR recovery was calculated as the decrease of HR per minute between the peak exercise period and 3 min post-exercise ((HRat peak-HR3 min post-exercise)/3). Those subjects who showed signs suggestive of myocardial ischemia were excluded from the analysis. Positive myocardial ischemia was defined as J point and ST80 (defined as the point that is 80 msec from the J point) depression of 0.1 mV or more and/or an ST-segment slope within the range of ±1 mV/sec in 3 consecutive beats. Tightly sealing breathing mask connected to an airflow sensor was used. Respiratory gas analysis was done by dynamic breath-by-breath measurement using JAEGER system (VIASYS Healthcare, Hoecherg, Germany). Various respiratory parameters including minute ventilation, oxygen uptake and carbon dioxide output were measured with a sampling interval of 8 sec to determine the maximal oxygen uptake.
All values were expressed as a mean±standard deviation. Comparisons of means between the 2 groups were done using a Student t-test. Bivariate correlations between variables were evaluated by Spearman correlation. A multiple regression model was constructed with HR recovery as the dependent variable, and independent variables were those variables showing significant association with HR recovery upon uni- and bivariate analysis. Both stepwise regression and empirical exploration were used for the final model selection. Criteria for stepwise regression were: 0.25 of probability to enter and 0.10 of probability to leave. Interactions between the independent variables were explored and insignificant interaction terms were removed from the final model. A p value <0.05 was considered statistically significant. SAS for Windows v. 8.01 (Cary, NC, U.S.A.) was used for the analysis.
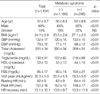
Demographic and laboratory characteristics of the population are shown in Table 1. The majority of the subjects had a sedentary lifestyle, with only 15% doing regular exercise more than 4 times per week. Twelve percent of the subjects reported that they were either hypertensive or on antihypertensive medications. Seventeen percent of the subjects were found to have metabolic syndrome, and their clinical and basic laboratory characteristics are shown in Table 1. Of the subjects with metabolic syndrome, 12.8% had 3, 4.4% had 4 and less than 1% had all 5 components of metabolic syndrome. As is shown in Table 1, these variables display significant difference between those with and without metabolic syndrome in the expected direction, except that the proportion of smokers was not different between the 2 groups. Their heart rates at rest, at the peak exertion time and at 3 min post-exercise were 62±9, 151±16 and 112±29 per minute, respectively.
HR recovery was lower in men (12.2±9.8 vs. 14.6±9.4 per minute for women, p<0.0001) and in smokers (10.7± 10.6 vs. 13.5±10.2 per minute for nonsmoker, p<0.0001) (Fig. 1). HR recovery inversely correlated to age, (r=-0.25,p<0.0001) resting SBP, (r=-0.23, p<0.0001) resting DBP, (r=-0.25, p<0.0001) and BMI, (r=-0.07, p<0.05). HR recovery correlated positively to HDL-cholesterol (r=0.12, p<0.0001) (Fig. 2). The resting HR and maximal oxygen uptake showed no correlation with HR recovery.
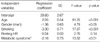
Subjects with metabolic syndrome had significantly impaired HR recovery as compared to those without metabolic syndrome (10.3±11.6 vs. 13.6±9.7 per min, p<0.0001, Fig. 1). The resting HR, a parameter reflecting the balance between sympathetic and parasympathetic tone in the basal state, was higher in the presence of metabolic syndrome (64.3±10.3 vs. 61.6±9.1 per min, p<0.0001). There was a statistically significant difference in HR recovery with increasing number of criteria of the metabolic syndrome present (p<0.05). The post-hoc multiple comparison showed that HR recovery was significantly different between those who had 0 and 1 factors, who had 0 and 3 factors, and who had 2 and 3 factors of metabolic syndrome (Fig. 3). By multivariate analysis, the presence of metabolic syndrome was independently associated with delayed HR recovery after an adjustment for age, gender, smoking and resting HR, which is a marker of sympathetic activity (p<0.01, Table 2). This result was not changed by analysis excluding 12% of subjects who were on any antihypertensive medication (p<0.01, data not shown).
This study demonstrates for the first time that subjects with metabolic syndrome show a delayed HR recovery as a suggested measure of vagal activity, and that HR recovery is delayed further in subjects having an increasing number of metabolic syndrome criteria met. This relationship was clear and persistent after we adjusted the results for several variables that can influence HR recovery, including the resting HR, thereby suggesting that there is a link between metabolic syndrome and impaired vagal activity, regardless of the presence of sympathetic overactivity.
Metabolic syndrome is a clinical concept that facilitates the identification of patients who have a metabolic derangement, this making them prone to atherosclerosis and thus, at risk for adverse cardiovascular events (18). Previous studies have suggested the relationship existing between autonomic dysfunction and hyperinsulinemia or insulin resistance. Obesity and associated hyperinsulinemia correlated with sympathetic overactivity, and this is reflected in the parameters of heart rate variability (10). Moreover, HR recovery after exercise was related to insulin sensitivity using the hyperinsulinemic eugleucemic clamp (13). These studies suggest that insulin resistance, which is thought to be an underlying abnormality for metabolic syndrome, is related to HR recovery. Recently, the association of metabolic syndrome with poor exercise capacity and poor heart rate recovery was demonstrated in patients who have established coronary heart disease (21). However, the relationship between metabolic syndrome and HR recovery after exercise has not been shown yet. We investigated a free-living population without cardiovascular disease or overt diabetes who agreed to take a health screening check up. A significant proportion of the subjects (17%) had metabolic syndrome, although less than 5% of them had obesity (BMI of over 30) and none had severe morbid obesity (BMI of over 40), and slightly less than half were just 'overweight' by Western standard. The average maximal oxygen uptake was 20.3 mg/kg/min, which seems to be quite poor for the average 51-yr-old person (19). A majority of subjects did not reach their 100% exercise performance performance level and it is highly likely that they were mostly sedentary, as judging from their frequency of sports activity, which might contribute to the development of metabolic syndrome.
The mechanism by which HR recovery is related to metabolic syndrome is largely a matter of speculation. One explanation involves the poorer aerobic fitness of the metabolic syndrome group. But in our data, the correlation between aerobic fitness and HR recovery is low. This result is probably because of the narrow range of maximal oxygen uptake, and the relationship between HR recovery and metabolic syndrome was persistent even after maximal oxygen uptake was forced into a multiple regression model (data not shown). Obesity, an important component of metabolic syndrome, is characterized by autonomic dysfunction in the parasympathetic system (12,22), which can be lessened by weight loss (23). There is also good evidence that the parasympathetic nervous system participates in the release of free fatty acids, thereby influencing insulin sensitivity and fat synthesis (24).
We did not make any sophisticated estimation of autonomic function, such as heart rate variability, but resting HR can be used as a crude estimate of sympathetic tone. Higher resting heart rate was shown to be associated with sympathetic overactivity, various cardiovascular risk factors including hypertension and higher fasting blood glucose (25,26) and mortality even after an adjustment for other risk factors (27,28). Our data showed, as was expected, that a higher resting HR is associated with metabolic syndrome, but it is not related to HR recovery. Delayed HR recovery was independently associated with metabolic syndrome after an adjustment for the resting HR. These findings suggest that metabolic syndrome is associated with impaired vagal reactivation in addition to the previously known relationship with sympathetic overactivity. When considering the abundance of robust data regarding the prognostic value of delayed HR recovery in various populations (1-6), and also our current findings of its association with metabolic syndrome, it is likely that impaired vagal tone as well as sympathetic overactivity contributes to the cardiovascular risk of metabolic syndrome. Aerobic fitness correlated to HR recovery (29) and it can be improved with exercise training, even in patients with existing cardiovascular disease (30). Exercise training is beneficial in overcoming various aspects of metabolic syndrome and improving the vagal tone may also be an important mechanism for benefiting from exercise training. The exercise protocols for measuring HR recovery were differently applied in previous studies. For example, Cole et al. (1) employed at least 2 min of cool-down period immediately after exercise which was considered as recovery phase, whereas Morshedi-Meibodi et al. (3) had patients to get off the treadmill immediately after peak exercise. Whether this difference influenced final results is not known. We used the protocol that allowed 30 sec of cool-down on treadmill after peak exercise to minimize the possible risk of blood pressure drop after sudden stopping of exercise (31).
Our study has a few limitations. About 12% of our subjects were on treatment with antihypertensive agents, and some of them may be on a drug that can interfere with the interpretation of HR response during stress tests such as beta-blockers. Because we can speculate that only a small proportion of our subjects were on beta-blockers, its influence on the final results of analysis was probably minimal. And the use of beta-blockers may not have a significant influence on the predictive value of HR recovery (32). Analysis excluding those on antihypertensive medication shows the same results in our study. Second, we did not have data on abdominal circumference, a better indicator of visceral fat than BMI alone (33). Substitution of BMI for abdominal circumference in defining metabolic syndrome might have had some influence on our results, but we consider BMI as a reasonable alternative having some predictive value for visceral adiposity (33). Finally, measurement of HR recovery can be done in various ways, but the arbitrary definition of HR decrease during the first minute after exercise has been frequently used because this parameter showed the predictive value for outcomes (1-3). Because we did not have the HR data at 1 min post-exercise, the slope of HR decrease during 3 min was used. In other studies, HR recovery at 2 min showed the maximal predictive value for mortality, but HR recovery at 3 min also had a significant predictive value (31,33,34). Therefore, it is not necessary to only look at average 1 min HR recovery as it assumes that the fall in heart rate after exercise is a simple linear function, which it is not. Some subjects had negative heart rate recovery, i.e., paradoxical heart rate increase during recovery period. While simple errors during data input is not likely because all the data were electronically transferred from exercise testing work station to research database, it is highly probable that extreme values such as -30/min is due to miscalculation of heart rate from RR interval by artifacts or ectopic beats. We were not able to reconfirm this error but such cases were very rarely seen in our data, and considering the sample size, this does not seem to influence the overall results. On the other hand, 'mild' negative value itself can be seen as in a previous study also reporting negative values in a minority of patients. For example, Morshedi-Meibodi et al. (3) reported that HR recovery ranges in lowest quintile are -10 to 8 and -2 to 20 per minute for men and women respectively. Other studies usually reported binary result of normal and abnormal HR recovery but did not show the actual range of HR recovery values. This unusual finding has not been discussed in detail before. As a speculation, those with very poor vagal reactivation may have actual increase of heart rate after exercise because plasma catecholamine during early recovery (about 90 sec) is actually higher than at peak exercise (35).
In conclusion, metabolic syndrome is significantly associated with impaired vagal reactivation. Therefore, cardiovascular risks associated with metabolic syndrome may also be mediated by the failure of vagal reactivation, in addition to sympathetic overactivity.
Figures and Tables
Fig. 1
Relationship between HR recovery, and gender, current smoking status, and the presence of metabolic syndrome. p<0.001 between each group.

Fig. 2
Correlation between HR recovery and individual parameters associated with metabolic syndrome.

Fig. 3
Delayed HR recovery and the number of factors indicating metabolic syndrome (mean-SD).*p<0.05 for post-hoc multiple comparison test, p value for overall difference between the group was <0.0001.

ACKNOWLEDGEMENT
We appreciate Dr Michael S. Lauer (Cleveland Clinic, U.S.A.) for criticism and kind comments on the paper.
References
1. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999. 341:1351–1357.

2. Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000. 132:552–555.

3. Morshedi-Meibodi A, Larson MG, Levy D, O'Donnell CJ, Vasan RS. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). Am J Cardiol. 2002. 90:848–852.

4. Kim HS, Lee JH, Kwon YS, Lee HS, Yang DH, Park HS, Jo YK, Chae SC, Jun JE, Park WH. Changes in heart rate during and after exercise treadmill test as prognostic factor in cardiovascular disease. Korean Circ J. 2004. 34:170–177.

5. Ju DU, Kang HJ, Kim SW, No TM, Son HS, Kang BJ, Kim SR, Lee BR, Jung BC, Lee JJ. The difference of heart rate recovery in ischemic heart disease comparing to normal. Korean J Med. 2004. 66:586–592.
6. Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003. 42:831–838.

7. Cheng YJ, Lauer MS, Earnest CP, Church TS, Kampert JB, Gibbons LW, Blair SN. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003. 26:2052–2057.

8. Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin. 2001. 19:369–387.

9. Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, Colucci WS. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989. 256:132–141.

10. Emdin M, Gastaldelli A, Muscelli E, Macerata A, Natali A, Camastra S, Ferrannini E. Hyperinsulinemia and autonomic nervous system dysfunction in obesity: effects of weight loss. Circulation. 2001. 103:513–519.
11. Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001. 14:304S–309S.

12. Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Engl J Med. 1988. 318:1077–1083.

13. Lind L, Andren B. Heart rate recovery after exercise is related to the insulin resistance syndrome and heart rate variability in elderly men. Am Heart J. 2002. 144:666–672.

14. Gottsater A, Ahmed M, Fernlund P, Sundkvist G. Autonomic neuropathy in type 2 diabetic patients is associated with hyperinsulinaemia and hypertriglyceridaemia. Diabet Med. 1999. 16:49–54.

15. Kawahara E, Ikeda S, Miyahara Y, Kohno S. Role of autonomic nervous dysfunction in electrocardiographic abnormalities and cardiac injury in patients with acute subarachnoid hemorrhage. Circ J. 2003. 67:753–756.

16. Lindmark S, Wiklund U, Bjerle P, Eriksson JW. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of type 2 diabetes patients and control subjects. Diabet Med. 2003. 20:399–405.

17. Laitinen T, Vauhkonen IK, Niskanen LK, Hartikainen JE, Lansimies EA, Uusitupa MI, Laakso M. Power spectral analysis of heart rate variability during hyperinsulinemia in nondiabetic offspring of type 2 diabetic patients: evidence for possible early autonomic dysfunction in insulin-resistant subjects. Diabetes. 1999. 48:1295–1299.

18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002. 106:3143–3421.
19. Ferguson C, Myers J, Foroelicher V. PD T, editor. Overview of exercise testing. Exercise and sports cardiology. 2001. 1st ed. New York: McGraw-Hill;92.
20. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehab Med. 1970. 2:92–98.
21. Spies C, Otte C, Kanaya A, Pipkin SS, Schiller NB, Whooley MA. Association of metabolic syndrome with exercise capacity and heart rate recovery in patients with coronary heart disease in the heart and soul study. Am J Cardiol. 2005. 95:1175–1179.

22. Scheurink AJ, Balkan B, Nyakas C, van Dijk G, Steffens AB, Bohus B. Energy homeostasis, autonomic activity and obesity. Obes Res. 1995. 3:Suppl 5. 721–727.

23. Rissanen P, Franssila-Kallunki A, Rissanen A. Cardiac parasympathetic activity is increased by weight loss in healthy obese women. Obes Res. 2001. 9:637–643.

25. Palatini P. Elevated heart rate as a predictor of increased cardiovascular morbidity. J Hypertens. 1999. 17:Suppl 3. 3–10.
26. Palatini P, Casiglia E, Pauletto P, Staessen J, Kaciroti N, Julius S. Relationship of tachycardia with high blood pressure and metabolic abnormalities: a study with mixture analysis in three populations. Hypertension. 1997. 30:1267–1273.
27. Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, Stamler J. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999. 149:853–862.

28. Mensink GB, Hoffmeister H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J. 1997. 18:1404–1410.

29. Darr KC, Bassett DR, Morgan BJ, Thomas DP. Effects of age and training status on heart rate recovery after peak exercise. Am J Physiol. 1988. 254:340–343.

30. Tiukinhoy S, Beohar N, Hsie M. Improvement in heart rate recovery after cardiac rehabilitation. J Cardiopulm Rehabil. 2003. 23:84–87.

31. Holtzhausen LM, Noakes TD. The prevalence and significance of post-exercise (postural) hypotension in ultramarathon runners. Med Sci Sports Exerc. 1995. 27:1595–1601.

32. Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001. 38:1980–1987.

33. Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002. 75:683–688.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download