Abstract
The effects of treatment with oral capecitabine vs. bolus 5-FU, administered concurrently with preoperative radiotherapy, were compared in the treatment of locally advanced rectal cancer (LARC). One hundred and twenty-seven patients with LARC received concurrent preoperative chemoradiation using two cycles bolus 5-FU (500 mg/m2/day) plus leucovorin (LV, 20 mg/m2/day) (Group I). Another LARC group received concurrent chemoradiation using two cycles 1,650 mg/m2/day of oral capecitabine and 20 mg/m2/day of LV (Group II, 97 patients). Radiation was delivered to the primary tumor at 50.4 Gy in both groups. Definitive surgery was performed 6 weeks after the completion of chemoradiation. A pathologic complete remission was achieved in 11.4% of patients in Group I and in 22.2% of patients in Group II (p=0.042). The down-staging rates of the primary tumor and lymph nodes were 39.0/68.7% in Group I and 61.1/87.5% in Group II (p=0.002/0.005). Sphincter-preserving surgery was possible in 42.1% of patients in Group I and 66.7% of those in Group II (p=0.021). Grade 3 or 4 leucopenia, diarrhea, and radiation dermatitis were statistically more prevalent in Group I than in Group II, while the opposite was true for grade 3 hand-foot syndrome. Preoperative chemoradiation using oral capecitabine was better tolerated than bolus 5-FU and was more effective in the promotion of both down-staging and sphincter preservation in patients with LARC.
In the treatment of locally advanced rectal cancer (LARC), the possible advantages of preoperative treatment compared with upfront surgery include lower toxicity, increased resectability, and an increased rate of sphincter preservation. A recently published phase III study in Germany showed improved pelvic control and sphincter preservation and less acute/chronic toxicity with preoperative chemoradiation than with postoperative chemoradiation (1). Although a randomised trial comparing chemoradiation to radiotherapy alone in the preoperative setting is not yet complete, the rationale for using concurrent chemotherapy is based on extrapolation from phase III postoperative trials (2, 3).
Regimens of 5-FU/leucovorin (LV) have been considered the standard therapy for patients with advanced colorectal cancer (4, 5). The LV-modulated intravenous (i.v.) bolus regimen is widely used because of its convenience and documented efficacy compared with bolus 5-FU alone (6). Comparing the efficacy of the two infusion methods, bolus vs. continuous infusion (CI) of 5-FU, CI is superior to bolus infusion in terms of tumor response and is associated with a slight increase in the overall survival of patients with advanced colorectal cancer (7). While CI has the biological advantage of prolonging the exposure of cells to 5-FU and improving anti-tumor activity, its disadvantages include the need for indwelling catheters and infusion pumps, with potential complications, such as infection, bleeding, thrombosis, and pneumothorax, which can result from central venous access (4).
Orally administered fluoropyrimidines were developed to prolong the anti-tumor activity of 5-FU. These drugs mimic the pharmacokinetics of CI 5-FU but avoid the technical barriers of i.v. infusion. In a questionnaire-based study, most patients preferred oral chemotherapy to i.v. infusion with respect to the quality of life, as long as the therapeutic effects were equivalent (8).
Capecitabine (Xeloda, Roche, Seoul, Korea) is a new oral fluoropyrimidine carbamate that was rationally designed to be converted to 5-FU, preferentially within tumor cells, via three sequential enzymatic steps. The enzyme thymidine phosphorylase converts 5'-deoxy-5-fluorouridine to 5-FU at the final step. In colorectal cancers, the level of thymidine phosphorylase is significantly higher in tumor tissue than in adjacent normal tissue or plasma. This results in the tumor-selective generation of 5-FU (9). In addition, radiotherapy can upregulate thymidine phosphorylase in tumor cells but not in normal tissue (10). Thus, there may be a synergistic effect between radiotherapy and capecitabine.
There has been no study comparing the efficacy of bolus 5-FU with the newly developed oral chemotherapeutic agent, capecitabine in the preoperative chemoradiotherapy of patients with rectal cancer. Beginning in 1993, however, we instituted a consistent treatment policy of preoperative chemoradiotherapy for patients with LARC. This policy comprised two different chemotherapeutic regimens: bolus 5-FU/LV and oral capecitabine/LV. The current study is aimed at retrospectively comparing the efficacies of these two regimens (including the degree of tumor down-staging and contribution to sphincter-preserving surgery) as well as their toxicities to patients in the preoperative chemoradiation therapy of LARC.
Since July 1993, patients with LARC were enrolled for preoperative chemoradiotherapy at Chungnam National University Hospital. The eligibility criteria were as follows: histological proof of rectal adenocarcinoma, tumor extension through the bowel wall (T3-T4) or pelvic lymph-node involvement without evidence of distant metastasis (as determined by clinical work-ups, including computed tomography), and a resectable or potentially resectable tumor. The pre-treatment clinical TNM stage was mainly determined by computed tomography (CT) imaging. Therefore, the pre-treatment TNM staging of the patients' tumors was carefully defined based on a joint review of CT images by a radiation oncologist, a surgeon, and a diagnostic radiologist. The TNM stages were reclassified according to the 5th edition of American Joint Committee on Cancer (AJCC) cancer staging manual. In general, the clinical T and N stages were determined by digital rectal examination and pelvic CT scans. Tumors were deemed T4 if there was evidence of invasion of neighboring organs, or T3 if palpation revealed the tumor to be partially or totally fixed, or if CT scanning showed tumor extension into the perirectal fat tissue. The lymph node involvement was regarded as positive when size of the adjacent perirectal lymph node was ≥3 mm or that of the other pelvic lymph node was ≥10 mm. Essential pre-treatment work-ups included a complete history, digital rectal examination, complete blood count, serum chemistry, CEA level, chest radiography, abdominal/pelvic CT scan, and colonoscopy with biopsy. Informed consent was obtained from all patients.
Between July 1993 and June 1999, 127 patients with LARC received two cycles of i.v. bolus 5-FU (500 mg/m2/day) and LV (20 mg/m2/day) for 5 days each. Each cycle of chemotherapy was administered concurrently in an outpatient setting on the first and fifth weeks of pelvic preoperative radiotherapy. These patients formed Group I (bolus-5-FU/LV-treated patients). Ninety-seven additional patients were registered from July 1999 to July 2002, at the time when the chemotherapy regimen had changed from i.v. 5-FU/LV to oral chemotherapy consisting of two cycles of capecitabine and LV. Capecitabine was given orally to these patients at a dose of 1,650 mg/m2/day, which was divided into two doses given 12 hr apart. LV (20 mg/m2/day) was also divided into two doses. In both group, LV was used to enhance of 5-FU activity. Patients were advised to take the oral chemotherapeutics after breakfast and dinner. One cycle of oral chemotherapy was continued for 14 days and was followed by a 7-day rest period. These patients formed Group II (oral capecitabine/LV-treated patients). The dose and administration schedule of capecitabine was based on results of the studies of Mackean et al. and Cassidy et al. (11, 12).
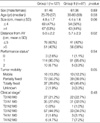
The characteristics of the patients enrolled in Groups I and II were analysed (Table 1). There was no significant difference between the two groups with respect to sex, age, and ECOG performance status. Regarding tumor mobility, Group II patients had more clinically fixed tumors than did patients in Group I, as determined by an initial digital rectal examination. The clinical stages of the tumors and lymph nodes were comparable in the two groups. The mean tumor size measured in the longest dimension by colonoscopy and/or CT imaging did not differ statistically between the groups. The mean distance of the tumor from the anal verge (clinically measured at the time of enrolment) was shorter in Group I patients than in Group II patients (5.0 vs. 5.7 cm). However there was no statistical difference between the two groups when only those patients who received surgery were analysed.
Throughout the 9-yr period that was studied, the radiation therapy technique administered to patients enrolled in the two groups was identical. Radiation was delivered with 6- and 10-MV photons using a three-field technique (posterior and both laterals) in most patients. Treatment was planned using computerised dosimetry, and a dose of 1.8 Gy per fraction was prescribed to cover the planning target volume with the 95% reference isodose (95% of the ICRU point dose). Patients were treated in the prone position and were encouraged to have a full bladder during irradiation. No devices were used to displace the small bowel from the treatment field, and shaped blocks were used to exclude normal tissues. Radiotherapy was delivered 5 days per week, once per day, at 1.8 Gy per day. The whole pelvis received 45 Gy in 25 fractions over 5 weeks; this was followed by a boost dose of 5.4 Gy administered in three fractions to the primary tumor by two lateral fields. For the whole-pelvis field, the superior border was at the L5-S1 interspace, and the inferior border was 3-4 cm below the primary tumor. The lateral border was 1.5 cm outside the true bony pelvis. For the lateral fields, the posterior margin was 1.5 cm behind the anterior bony sacral margin, and anterior border was usually anterior or midacetabulum.
During the course of radiation therapy, patients were evaluated and interviewed weekly with radiation oncologist in order to not only assess acute toxicity and compliance with the chemoradiation but also to verify that oral capecitabine was taken properly. Acute toxicity was assessed according to the NCI common toxicity criteria (13). Four weeks after the completion of chemoradiation, tumor-restaging procedures were performed that included physical examination, complete blood count, serum chemistry, CEA level, chest radiography, and abdominal/pelvic CT scan. Approximately 6 weeks after completion of the chemoradiation, patients underwent definitive surgery. Surgical management included a sphincter-preservation approach whenever possible, using the total mesorectal excision technique. Pathological evaluation of the surgical specimens, including the primary tumor and the removed nodes, was performed based on the tumor regression grade according to the criteria proposed by Mandard et al. for esophageal carcinomas treated with chemoradiotherapy (14). Only the cases showing the complete absence of residual tumor cells were designated as being in pathologic complete remission (pCR). In order to evaluate the effect of preoperative chemoradiation on tumor down-staging, the pre-treatment clinical TNM stage was compared with the postoperative pathological TNM stage. Primary tumor and node down-staging were defined as reductions in T and N stages by at least one level.
The patients' characteristics, hematologic/non-hematologic toxicities, down-staging, sphincter preservation, and postoperative complications were compared across the two groups using the chi-square test, Fisher's exact test, or the t-test when appropriate. p values of less than 0.05 were considered to be statistically significant. All statistical analyses were conducted using the SPSS (Version 10.0) statistical software program (SPSS, Chicago, IL, U.S.A.).
Among the 127 patients in Group I, treated with bolus 5-FU/LV, six had a modification of the chemotherapy scheme during the course of treatment: the second course was skipped in four patients and the dose was reduced in two patients because of toxicity. Only one of the 97 patients in Group II skipped the second course of oral chemotherapy, because of grade 2 nausea/vomiting along with the patient's refusal. After completing preoperative chemoradiation, 105 (82.7%) of the 127 patients in Group I and 90 (92.8%) of the 97 patients in Group II underwent definitive surgery. Twenty-two patients in Group I and seven patients in Group II refused definitive surgery following chemoradiation therapy. The desire for the preservation of a functional anus accounted for a considerable portion of the reasons expressed by these patients (14/22 in Group I, 5/7 in Group II). Two patients in Group I refused surgery; one experienced grade 3 diarrhea and leucopenia during chemoradiation, and the other experienced colitis after chemoradiation. The types and numbers of surgical resections performed in Groups I/II were: low anterior resection, 62/57; abdominoperineal resection, 28/11; colo-anal reconstruction, 5/18; Hartmann procedure, 4/2; total proctocolectomy, 6/0; and trans-anal full-thickness excision, 0/2.
Postoperative pathologic stages were evaluated in patients who underwent definitive surgery (Table 2). The pathologic stage of tumors in the patients of Groups I/II were as follows: pCR (T0N0) in 11.4/22.2%; stage I (T1-2N0) in 20.0/22.2%; stage II (T3-4N0) in 27.6/31.2%; stage III (any T, N1-2) in 31.5/21.1%; and stage IV (any T, any N, M1) in 9.5/3.3%. The overall distributions of postoperative pathological stages between Group I and Group II showed only marginal statistical difference (p=0.076). Especially with respect to pCR following preoperative chemoradiation, Group II patients had a higher pCR rate than Group I patients (p=0.042).
Postoperative pathological stages were analysed in both groups and was compared with pre-treatment clinical stages. Primary tumor and node down-staging were achieved in 39.0/61.1% and 68.7/87.5% of the patients in Groups I/II, respectively (Table 3). The overall down-staging rate, including primary tumor and node down-staging in Group I/II patients, was 70.5/86.7%. Group II patients had a higher overall down-staging rate than patients in Group I (p=0.007).
In this study, one colorectal surgeon operated on all but five of the 195 patients. Sphincter preservation was analysed in patients with distal rectal tumors, defined as those initially located 5 cm or less from the anal verge (Table 3). Fifty-seven (54.3%) of 105 patients in Group I and 36 (40%) of 90 patients in Group II had tumors located 5 cm or less from the anal verge on initial enrolment. The postoperative sphincter-preservation rates of patients with distal rectal tumors were 24/57 (42.1%) in Group I and 24/36 (66.7%) in Group II (p=0.021).
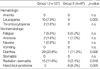
The incidences of acute toxicities during chemoradiation in Groups I and II are listed in Table 4. With respect to grade 3 or 4 hematologic toxicity, ten patients in Group I had grade 3 (in 8) or grade 4 (in 2) leucopenia, but none of the patients in Group II had grade 3 or 4 leucopenia (p=0.005). Among Group I patients, one had grade 3 and another had grade 4 thrombocytopenia, whereas no thrombocytopenia of grade 3 or 4 developed in Group II. Regarding non-hematologic toxicities, no grade 4 toxicities were noted in either group. The most common grade 3 non-hematologic toxicity in Group I patients was diarrhea (22.8%), followed by radiation dermatitis (11.8%) and fatigue (5.5%). Group II patients had the following grade 3 non-hematologic toxicities: diarrhea (11.3%), hand-foot syndrome (6.2%), and fatigue (5.2%). Grade 3 diarrhea and radiation dermatitis were more prevalent in Group I, but grade 3 hand-foot syndrome occurred more frequently in Group II (p=0.005). There were no life-threatening complications associated with either of the chemoradiation regimens and no postoperative deaths.
Postoperative complications occurring within 3 months after surgery were evaluated. In Group I patients, postoperative complications requiring conservative management were delayed wound healing over 4 weeks in four, intestinal obstruction in four, neurogenic bladder in one, and pelvic abscess in one. Three complications required surgical management in Group I patients: intestinal obstruction in one, anastomosis leakage in one, and pelvic abscess in one. In Group II patients, postoperative complications requiring conservative management were delayed wound healing in one, intestinal obstruction in six, ureter stenosis in one, neurogenic bladder in one, and pelvic abscess in two. Complications requiring surgical management in Group II patients were anastomosis leakage in one, pelvic abscess in one, and rectovaginal fistula in one. There was no statistical difference in the rate of postoperative complications between the patients in Group I (12.4%) and Group II (15.5%) (p=0.81).
Over the past decade, several groups have reported the effects of combining radiotherapy with 5-FU as a bolus or CI. The rates of pathologic complete response have been between 8% and 33% (1, 15-18). Although the data are limited and there are no studies comparing bolus versus CI delivery, the efficacy results, including pathologic complete response, down-staging, and sphincter preservation, appear to favour CI schemes. However, the need for indwelling catheters and infusion pumps imposes limits on this method of administration.
During a 9-yr period, we had consistently employed a treatment policy of concurrent preoperative chemoradiotherapy (differing only in chemotherapy regimen: bolus 5-FU/LV vs. oral capecitabine/LV) for patients with LARC. As the pretreatment characteristics of the patients in the two groups were nearly comparable, we retrospectively compared acute toxicities during preoperative chemoradiotherapy, the pathologic responses of the tumors, and sphincter preservation.
Patients treated with CI 5-FU tend to experience lower overall levels of toxicity than those receiving bolus chemotherapy. These differences are especially apparent for hematologic toxicities (19). The main toxicities of bolus 5-FU injection are oral mucositis, gastrointestinal toxicity (diarrhea), myelosuppression, and skin toxicity. In a randomised phase III trial, capecitabine was less toxic than bolus 5-FU/LV when administered to patients with advanced colorectal cancer (20). Oral capecitabine treatment results in a significantly lower incidence of myelosuppression, diarrhea, stomatitis, nausea, and alopecia than bolus 5-FU/LV treatment. Grade 3 hand-foot syndrome developed more frequently after oral capecitabine treatment than after bolus 5-FU/LV treatment. In patients receiving high-dose CI 5-FU/LV, hand-foot syndrome was a frequent side effect, but it did not significantly affect the patients' quality of life or the delivery of the planned chemotherapy (21). In this study, grade 3/4 leucopoenia and thrombocytopenia occurred only in the bolus 5-FU/LV group, with no incidences of these toxicities in the oral capecitabine/LV group. The most commonly occurring grade 3 non-hematologic toxicities were diarrhea and radiation dermatitis in the bolus 5-FU/LV treatment group, and diarrhea and hand-foot syndrome in the oral capecitabine/LV group. Statistically, there was less diarrhea and radiation dermatitis in the latter group, although hand-foot syndrome occurred more frequently. As the tumors in Group I patients were located slightly nearer to the anal verge than those in Group II patients, this would have contributed, to some extent, to the clinically significant radiation dermatitis in Group I, especially because a perineal reaction to radiation therapy would be severe in patients with distal rectal tumors. Most patients with symptomatic hand-foot syndrome in the oral capecitabine/LV group could be easily managed with vitamin B6 administration and supportive care without interrupting the radiotherapy schedule.
Tumor down-staging induced by preoperative chemoradiotherapy is closely related to pathologic complete response and sphincter preservation in patients with distal rectal cancer. Janjan et al. examined the rates of tumor down-staging and sphincter preservation after CI 5-FU in 117 patients with LARC (16). The patients received radiotherapy of 45 Gy over 5 weeks concurrent with CI 5-FU (300 mg/m2/day). The rate of tumor down-staging was 62%, and the rate of pathologic complete response was 27%. Crane et al. also reported that, in clinical T3/4 rectal cancers, the addition of CI 5-FU to preoperative radiotherapy increased tumor down-staging (62 vs. 42%), pathologic complete response (23 vs. 5%), and sphincter preservation (39 vs. 13%) in the subset of patients who had tumors within 6 cm of the anal verge (17). Our results of tumor down-staging and pathologic response in the oral capecitabine/LV group are compatible to these findings. The higher down-staging rate of primary tumors in oral capecitabine group than in LV group may explain the higher sphincter-preservation rate in patients with distal rectal tumors in our study. However, favourable effects, including tumor down-staging and sphincter preservation, found in the oral capecitabine group may have been affected by several factors, such as an inherent weakness in the retrospective study, a difference in the tumor distance from the anal verge, or pre-treatment clinical TNM staging made without endorectal ultrasound or MRI. A pathologic response following preoperative chemoradiotherapy of patients with LARC can be regarded as a significant prognostic indicator for long-term survival. Mohiuddin et al. reported that the degree of down-staging (postoperative pathologic stage) was closely related to long-term survival (100% for pT0-2N0, 80% for pT3-4N0, and 73% for pTxN1-2) (15). This suggests that a higher pathologic response, induced by an effective preoperative chemoradiotherapy, can be translated into a better survival outcome of patients with LARC.
Although there are few randomised studies comparing bolus 5-FU with CI 5-FU in the preoperative radiotherapy of rectal cancer, we can postulate that CI 5-FU is superior to bolus 5-FU in terms of tumor response based on other studies of advanced colorectal cancer (7). As oral capecitabine mimics CI 5-FU in its pharmacologic action in vivo, it is not surprising that patients treated with oral capecitabine/LV had higher rates of tumor down-staging, pathologic complete response, and sphincter preservation than did patients treated with bolus 5-FU/LV in this study.
In summary, our results suggest that preoperative chemoradiation with oral capecitabine/LV is more tolerable with respect to side effects and offers a more effective treatment modality than bolus 5-FU/LV, as measured by tumor down-staging, pathologic response, and sphincter preservation.
Figures and Tables
References
1. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004. 351:1731–1740.

2. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985. 312:1465–1472.
3. Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991. 324:709–715.

4. Grem JL. Systemic treatment options in advanced colorectal cancer: perspectives on combination 5-fluorouracil plus leucovorin. Semin Oncol. 1997. 24:Suppl 18. S18-8–S18-18.
6. Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989. 7:1407–1418.

7. Meta-analysis Group In Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998. 16:301–308.
8. Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997. 15:110–115.

9. Schüller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B. Preferential activation of capecitabine in tumor following oral administration in colorectal cancer patients. Cancer Chemother Pharmacol. 2000. 45:291–297.
10. Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999. 5:2948–2953.
11. Mackean M, Planting A, Twelves C, Schellens J, Allman D, Osterwalder B, Reigner B, Griffin T, Kaye S, Verweij J. Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol. 1998. 16:2977–2985.

12. Cassidy J, Dirix L, Bissett D, Reigner B, Griffin T, Allman D, Osterwalder B, Van Oosterom AT. A phase I study of capecitabine in combination with oral leucovorin in patients with intractable solid tumors. Clin Cancer Res. 1998. 4:2755–2761.
13. Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, Shipley W, Curran W. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000. 47:13–47.

14. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Cancer. 1994. 73:2680–2686.
15. Mohiuddin M, Hayne M, Regine WF, Hanna N, Hagihara PF, Mc-Grath P, Marks GM. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000. 48:1075–1080.

16. Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R, Rich TA, Skibber J. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M.D. Anderson cancer center experience. Int J Radiat Oncol Biol Phys. 1999. 44:1027–1038.
17. Crane CH, Skibber JM, Birnbaum EH, Feig BW, Singh AK, Delclos ME, Lin EH, Fleshman JW, Thames HD, Kodner IJ, Lockett MA, Picus J, Phan T, Chandra A, Janjan NA, Read TE, Myerson RJ. The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2003. 57:84–89.

18. Mehta VK, Poen J, Ford J, Edelstein PS, Vierra M, Bastidas AJ, Young H, Fisher G. Radiotherapy, concomitant protracted-venous-infusion 5-fluorouracil, and surgery for ultrasound-staged T3 or T4 rectal cancer. Dis Colon Rectum. 2001. 44:52–58.
19. Thrall MM, Wood P, King V, Rivera W, Hrushesky W. Investigation of the comparative toxicity of 5-FU bolus versus 5-FU continuous infusion circadian chemotherapy with concurrent radiation therapy in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2000. 46:873–881.

20. Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001. 19:2282–2292.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download