Abstract
Interferon regulatory factor 7 (IRF7) is one of the transcriptional factors for the activation of type I Interferon (IFN) genes. It is known that IRF7 and the latent membrane protein 1 (LMP1) of Epstein-Barr virus (EBV) are highly expressed in EBV type III latency cells, and LMP1 induces mRNA expression of IRF7. In this study, the expression pattern of endogenous IRF7 was observed in several B cell lines with or without EBV infection by immunofluorescence staining. IRF7 was localized in the cytoplasm of EBV-negative B cells and EBV type I latency B cell lines. However, IRF7 was located both in the cytoplasm and nucleus of EBV type III latency cell lines. In the Jijoye cell (type III latency cell), IRF7 was colocalized with LMP1 in the cytoplasm in a capping configuration, and their interaction was confirmed by co-immunoprecipitation of LMP1 and IRF7. This colocalization was confirmed by co-transfection of IRF7 and LMP1 plasmids in EBV-negative B cells. These results suggest that the IRF7 and LMP1 interact with each other, and this may relate to the mechanism whereby LMP1 exerts functional effects in B-lymphocytes.
Epstein-Barr virus (EBV) immortalizes human B-lymphocytes in vitro; and latent membrane protein 1 (LMP1) of the EBV is essential for this process (1). LMP1 is a membrane protein composed of a short cytoplasmic amino-terminus linked to a transmembrane domain, with six membrane-spanning segments connected with short turns, and a long cytoplasmic carboxy-terminal domain (2). The six transmembrane domains of LMP1 play an important role for the aggregation of LMP1 and in forming a patch, which is essential for the activation of the signaling pathway (3). LMP1 associates with vimentin and other cellular proteins, forming a patch in the lymphocyte plasma membrane. LMP1 also colocalizes with the LMP2A protein of EBV, which recruits cellular protein tyrosine kinases (4,5). Biological effects of LMP1, such as induction of several cellular genes, are mediated by the activation of several signaling pathway, such as NF-κB, p38/MAPK, Jak/STAT, JNK/AP-1, PI3K/Akt etc. (6).
Interferon regulatory factors (IRFs) are a growing family of transcriptional factors that share homology within the amino-terminal DNA binding domain (115 amino acids) (7). They are implicated in several biological processes, such as antiviral defense, cytokine signaling, cell growth, differentiation, oncogenesis and apoptosis (8-11). Two members of IRF family, IRF3 and IRF7, are considered to be important factors for the expression of type I interferon (IFN) genes following viral infection (12). IRF3 is constitutively expressed in a variety of cells and tissues (9), but IRF7 is expressed predominantly in lymphoid cells (13,14). IRF7 locates in the cytoplasm and translocates to the nucleus after virus infection, via phosphorylation by IKKε(14-16). Recently, it was found that IRF7 and LMP1 induce each other within a regulatory circuit and IRF5 brakes the IRF7/LMP1 circuit (17,20). IRF7 can induce expression of LMP1 by binding to the IFN-stimulated response element (ISRE) of the LMP1 promoter, but the mechanism of induction and phosphorylation of IRF7 by LMP1 was unknown yet (19). LMP1 protein is a membranes protein and LMP1 cannot bind to the IRF7 promoter directly. Therefore, the interaction between LMP1 and IRF7 or other mediators might need for the regulation of IRF7 expression by LMP1.
Thus, this study was designed to determine the localization of endogenous IRF7 and the physical interaction of IRF7 with LMP1 in B lymphoblastoid cell lines.
All the cell lines used in this experiment were maintained in RPMI 1640 medium containing 10% fetal bovine serum and antibiotics (Gibco-BRL, CA, U.S.A.). DG75 is an EBV-negative Burkitt's lymphoma cell line (21). Akata and Sav I cell lines are EBV-positive type I latency B cell lines (22). Jijoye and Sav III are EBV-positive type III latency B cell lines (23,24).
The expression vector used in this study is pcDNA3. The LMP1 expression plasmid, pcLMP1 (a gift from Tomakazu Yoshizaki), the IRF7 expression plasmid, pcIRF7 (13), and FLAG-tagged LMP1 wild-type (WT), (a gift from Nancy Raab-Traub), were used for transient transfection.
DG75 cells were transfected with pcDNA3, pcIRF7, or FLAG-tagged wild type LMP1. 1×107 cells in 0.5 mL medium were pulsed once at 320 V, 975 µF capacitance in a 0.4 cm cuvette in Gene Pulse II (Bio-Rad, Hercules, CA, U.S.A.) with a total of 10 µg of DNA plasmids. Cells were harvested 48 hr after transfection and used for immunofluorescence assay, Western blot and co-immunoprecipitation.
Anti-EBV LMP CS1-4 clone (DAKO, Denmark), anti-IRF7 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), and anti-M2 Flag antibody (Sigma, St. Louis, MO, U.S.A.) were used as primary antibodies. Anti-mouse Ig-HRP, anti-rabbit Ig-HRP (Amersham, U.K.), and anti-mouse kappa-HRP (Southern Biotechnology Associates, Birmingham, AL, U.S.A.) were used as the secondary antibodies for Western blot or immunoprecipitation. Donkey anti-rabbit-FITC, donkey anti-mouse-rhodamine (Jackson ImmunoResearch Laboratory, West grove, PA, U.S.A.) were used as the secondary antibodies for immunofluorescence assay.
Jijoye and transfected DG75 cells were harvested and washed twice in phosphate-buffered saline (PBS). The cells were smeared on the HTC well slides (Cel-line, Portmouth, NH, U.S.A.) and air-dried. Cells were fixed with cold acetone for 10 min, and then blocked with 5% normal donkey serum (Jackson Laboratory, PA, U.S.A.) for 30 min at 37℃. Primary antibodies were incubated for 90 min at 37℃. Anti-EBV LMP CS1-4 clone and anti-IRF7 antibody were diluted 1:50 in PBS containing 2% normal donkey serum. Slides were washed three times with PBS. Secondary antibodies were diluted 1:100 in PBS and incubated for 45 min at 37℃. After washing with PBS, they were mounted using Vector-shield (Vector Laboratory, Burlingame, CA, U.S.A.), and then examined with the Axioscope fluorescence microscope (Zeiss, Germany) or the TCS-NT confocal microscope (Leica, Germany). The images were captured using a Scion software program.
Jijoye cells (4×107 cells per immunoprecipitation) were washed in cold PBS twice and then lysed in 900 µL of lysis buffer (20 mM HEPES, 0.5% Nonidet P-40 [NP-40], 250 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], sodium-orthovanadate, sodium fluoride, 1 tablet of complete [Amersham]) for 20 min at 4℃. The supernatant was clarified by centrifugation for 15 min at 4℃. IRF7 and LMP1 proteins were immunopurified with 20 µL of anti-IRF7 antibody with 100 µL of protein A Sepharose (Sigma) or 20 µL anti-LMP1 antibody with 100 µL of protein G Sepharose (Sigma), respectively, for overnight incubation with constant rocking at 4℃. For DG75 cells, cell lysates were immunopurified with 100 µL of anti-FLAG M2 agarose affinity gel (Sigma) for >4 hr with constant rocking at 4℃, or with 20 µL of anti-IRF7 antibody with 100 µL protein A Sepharose for overnight incubation with constant rocking at 4℃. Immunocomplexes were washed 4 times in lysis buffer, diluted in 2×sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min and then run on 10% SDS-polyacrylamide gel. LMP1 and IRF7 proteins were detected by immunoblotting as described below.
Immunoprecipitated samples separated on SDS-PAGE were transferred to nitrocellulose membranes using a semidry transfer apparatus. The membranes were blocked in PBS containing 10% nonfat dry milk and then probed with anti-IRF7 monoclonal antibody (1:50 dilution), anti-M2 flag antibody (1:500 dilution) in an overnight incubation at 4℃, or with anti-LMP1 monoclonal antibody (1:50 dilution) for 2 hr at room temperature (RT). The membranes were then washed three times with PBS containing 0.1% Tween 20, incubated with corresponding horseradish peroxidase (HRP)-linked secondary antibodies for 1 hr at RT, and reactivity was detected using a chemiluminescence reagent (Pierce, Rockford, IL, U.S.A.).
DG75, Akata, Sav I, Sav III, and Jijoye cell lines were used for the immunofluorescence staining to confirm the localization of endogenous IRF7 protein. Immunoreactivity for IRF7 is shown as small bright spots in the EBV-negative (Fig. 1A) and EBV-positive type I latency cell lines (Fig. 1B, C). However, the IRF7 distributes as scattered granules throughout the entire cell of type III latency Sav III cells (Fig. 1D). There were two patterns of IRF7 in Jijoye, one identical with the EBV negative or type I latency cell line (Fig. 1E), and the other consists of clumps and scattered granules in the cells (Fig. 1F).
To verify the localization of LMP1 and IRF7 proteins, double immunofluorescence was performed in Jijoye and Sav III cells, which express endogenous LMP1 and IRF7. The pattern of LMP1 immunofluorescence was identical with previous reports (2,6) and IRF7 colocalized with LMP1 as a capping configuration in the cytoplasm of Jijoye cells (Fig. 2A, B). There was only weak co-localization in Sav III cells (Fig. 2C). DG75 cells were transiently co-transfected with LMP1 and IRF7 plasmids and these are colocalized in DG75 cells, which expresses both of the proteins (Fig. 2D).
To confirm the physical interaction of the endogenous LMP1 and IRF7 proteins, co-immunoprecipitation was performed in the Jijoye cell. LMP1 and IRF7 were immunopurified with monoclonal CS1-4 antibody or anti-IRF7 antibody, and the immune complexes were subjected to SDS-PAGE and then analyzed by Western blotting (Fig. 3). Both LMP1 and IRF7 were detected by Western blot in the immunocomplexes, which were immunopurified with LMP1 antibody (Fig. 3, lane 1-2), or IRF7 antibody (Fig. 3, lanes 3-4). This result suggests that endogenous LMP1 and IRF7 interact with each other in the Jijoye cell. FLAG-LMP1 (WT), IRF7, or both of FLAG-LMP1 (WT) and IRF7 were over-expressed in DG75 cells by electroporation using Gene Pulser II, followed by co-immunoprecipitation. Cell lysates were immunopurified with M2 flag antibody or rabbit polyclonal IRF7 antibody. LMP1 and IRF7 were detected in the immune complex, which was purified with M2 flag antibody (Fig. 4A), the cell lysate that was co-transfected with both LMP1 and IRF7 (Fig. 4A, lane 4). In the immune complex purified with IRF7 antibody (Fig. 4B), LMP1 was detected in both cell lysates transfected with LMP1 only (Fig. 4B, lane 2), or LMP1 plus IRF7 (Fig. 4B, lane 4).
IRF7 is a member of the interferon regulatory factor family. It is predominantly expressed in cells of lymphoid origin (13,14). IRF7 was first identified as a repressor of the Bam HI Q promoter (Qp) of EBV, which is used for the transcription of the Epstein-Barr nuclear antigen 1 (EBNA1) mRNA in type I latency cells (13). It is known that IRF7 and IRF3 are important for the induction of type I IFN gene expression following viral infection, and that IRF7 gene expression is induced by both type I IFN and by viral infection (12,14,25,26). As with IRF3, it is considered that the un-phosphorylated IRF7 (inactive form) locates in the cell cytoplasm and the phosphorylated IRF7 (active form) translocates to the nucleus (14,15). However, another report suggests that un-phosphorylated IRF7 is present in both the cytoplasm and the nucleus (27). In all of these previous experiments, the over-expression systems of IRF7 fusion proteins were used for the localization of IRF7. As mentioned in a recent paper concerning the half-life of IRF7 (18), there is a possibility that over-expressed IRF7 fusion proteins might differ from endogenous IRF7.
To confirm the localization of endogenous IRF7, we applied immunofluorescent staining to several B-lymphoblastoid cell lines. There are two visual patterns of endogenous IRF7, one of tiny spots within a large granule located in the cytoplasm near the nuclear indentation, and the other of scattered granules in the nucleus with clumps in the cytoplasm (Fig. 1). The former pattern was found in EBV-negative and type I latency B-lymphoblastoid cell lines and is considered to represent an inactive state of IRF7 (Fig. 1A-C). The latter pattern was found in type III cell lines (Fig. 1D, F) and considered to represent an active state of IRF7.
The expression of IRF7 protein was very low in EBV-negative and type I latency B-lymphoblastoid cell lines (Fig. 1A-C), but it was high in the Sav III cell line (Fig. 1D). These results are in agreement with a previous Western blot results (13). In Jijoye a type III latency cell line, both of these patterns were found (Fig. 1E, F), and Raji (a type III latency lymphoblastoid cell line) also showed two patterns of IRF7 similar to those found in Jijoye cells (data not shown). There was evidence that LMP1 and IRF7 have a strong relationship in EBV-positive B cell lines (13,17,19). In type I latency, LMP1 is not expressed and IRF7 expression is very low, but the expression of both LMP1 and IRF7 are high in type III latency (13). It is interesting that even though LMP1 protein is highly expressed in Sav III and Jijoye cell lines in Western blot, there are two patterns of IRF7 expression in Jijoye cells when detected by immunofluorescence staining. Liebowitz et al. reported that about 2% of the Jijoye cells showed evidence of early or late viral replication (3). Therefore, some of Jijoye cells are not actually type III latency cells and they do not express LMP1. Not only Jijoye, we found that some of Sav III and Raji cells also did not express LMP1 in immunofluorescence staining (data not shown). Cell proliferation also influenced to the EBV replication and interferons also regulate the expression of interferon regulatory factor 7 (25). Those viral or cellular factors may be involved in the induction and/or activation of IRF7 protein. Further experiments will be necessary to confirm which additional factors are involved in the regulation of IRF7.
In double immunofluorescence staining, colocalization of LMP1 and IRF7 was detected in the cytoplasm of Jijoye cells (Fig. 2) and interaction was also confirmed by co-immunoprecipitation (Fig. 3). The functional diversity of such a transcriptional factor is dependent on its modification, such as by phosphorylation and/or interactions with other transcriptional factors that are co-expressed and/or activated in the cell (27). LMP2A also colocalizes in the LMP1 patch, and LMP2A recruits several cellular kinases, which are involved in the B cell signaling pathway (5). LMP1 and IRF7 over-expression in EBV negative DG75 cells showed that they interacted physically (Fig. 4). The reason why the LMP1 detected in the second lane (Fig. 4B upper panel) is may be that over-expressed LMP1 interacts with endogenous IRF7.
These results suggest that interaction between LMP1 and IRF7 may be necessary for the signal transduction pathway of LMP1 and/or IRF7. Further experiments will be needed in order to provide additional details about the relationship between LMP1 and IRF7 in the signaling pathway of Epstein-Barr virus infected B-lymphocytes.
Figures and Tables
Fig. 1
The immunofluorescence pattern of endogenous IRF7 in various B lymphoblastoid cell lines. Immunofluorescence assays (IFA) were performed with a rabbit polyclonal IRF7 antibody, with subsequent reaction with donkey anti-rabbit IgG-FITC in DG75 (A), Akata (B), Sav I (C), Sav III (D) and Jijoye (E, F) cell lines. IRF7 was captured with a Axioscope fluorescence microscope using a Scion software program.
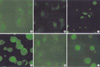
Fig. 2
Colocalization of LMP1 and IRF7. The LMP1 and IRF7 proteins are double immunostained with mouse monoclonal LMP1 antibody and rabbit polyclonal IRF7 antibody, and subsequently reacted with donkey anti-mouse IgG-rhodamine and donkey anti-rabbit IgG-FITC, respectively. Panel (A) and (B) are Jijoye, (C) is Sav III, and (D) is DG75 cell line co-transfected with LMP1 and IRF7 plasmids. Fluoresecence was captured with a confocal microscope. Red and green color represent of LMP1 and IRF7, respectively; yellow indicates colocalization of both proteins.

Fig. 3
In vivo association of LMP1 with IRF7 in Jijoye. Cell lysates of Jijoye were subjected to immunoprecipitation with normal mouse serum (lane 1), LMP1 antibody (lane 2), normal rabbit serum (lane 3), or IRF7 antibody (lane 4). The immunocomplexes were analyzed by SDS-PAGE on 10% gel and subjected to western blot analysis with mouse LMP1 antibody (upper panel) or rabbit IRF7 antibody (lower panel).

Fig. 4
Interaction of LMP1 and IRF7 in transfected DG75. DG75 cells were transfected with pcDNA3 (lane 1), FLAG-pcLMP1 (lane 2), pcIRF7 (lane 3) or FLAG-pcLMP1 plus pcIRF7 (lane 4). Cell lysates were immunopurified with M2 FLAG antibody (A) or IRF7 antibody (B) and then subjected to western blot analysis with mouse LMP1 antibody (upper panels) or rabbit IRF7 antibody (lower panels).

References
1. Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993. 90:9150–9154.

2. Hennessy S, Fennewald S, Hummel M, Cole T, Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci USA. 1984. 81:7207–7211.

3. Liebowitz D, Wang D, Kieff E. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J Virol. 1986. 58:233–237.

4. Longnecker R, Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990. 64:2319–2326.

5. Longnecker R, Druker B, Roberts TM, Kieff E. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J Virol. 1991. 65:3681–3692.

6. Li HP, Chang YS. Epstein-Barr virus latent membrane protein 1: structure and functions. J Biomed Sci. 2003. 10:490–504.

7. Nguyen H, Hiscott J, Pitha PM. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997. 8:293–312.

8. Kimura T, Nakayama K, Penninger J, Kitagawa M, Harada H, Matsuyama T, Tanaka N, Kamijo R, Vilcek J, Mak TW, Taniguchi T. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science. 1994. 264:1921–1924.

9. Au WC, Moore PA, Lowther W, Juang YT, Pitha PM. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc Natl Acad Sci USA. 1995. 92:11657–11661.

10. Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993. 259:971–974.

11. Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier MS, Aizawa S, Mak TW, Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994. 77:829–839.

12. Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000. 13:539–548.
13. Zhang L, Pagano JS. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997. 17:5748–5757.

14. Au WC, Moore PA, LaFleur DW, Tombal B, Pitha PM. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998. 273:29210–29217.

15. Lin R, Mamane Y, Hiscott J. Multiple regulatory domains control IRF-7 activity in response to virus infection. J Biol Chem. 2000. 275:34320–34327.

16. Sharma S, tenOver BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003. 300:1148–1151.

17. Zhang L, Pagano JS. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J Virol. 2000. 74:1061–1068.

18. Zhang L, Wu L, Hong K, Pagano JS. Intracellular signaling molecules activated by Epstein-Barr virus for induction of interferon regulatory factor 7. J Virol. 2001. 75:12393–12401.

19. Ning S, Hahn AM, Huye LE, Pagano JS. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J Virol. 2003. 77:9359–9368.

20. Ning S, Huye LE, Pagano JS. Interferon regulatory factor 5 represses expression of the Epstein-Barr virus oncoprotein LMP1: braking of the IRF7/LMP1 regulatory circuit. J Virol. 2005. 79:11671–11676.

21. Ben-Bassat H, Goldblum N, Mitrani S, Goldblum T, Yoffey JM, Cohen MM, Bentwich Z, Ramot B, Klein E, Klein G. Establishment in continuous culture of a new type of lymphocyte from a "Burkitt like" malignant lymphoma (line D.G.-75). Int J Cancer. 1977. 19:27–33.
22. Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991. 5:147–156.

23. Ragona G, Ernberg I, Klein G. Induction and biological characterization of the Epstein-Barr virus (EBV) carried by the Jijoye lymphoma line. Virology. 1980. 101:553–557.

24. Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996. 70:623–627.

25. Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998. 17:6660–6669.

26. Au WC, Yeow WS, Pitha PM. Analysis of functional domains of interferon regulatory factor 7 and its association with IRF-3. Virology. 2001. 280:273–282.

27. Marie I, Smith E, Prakash A, Levy DE. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Mol Cell Biol. 2000. 20:8803–8814.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download