Abstract
We report a rare case of giant vascular eccrine spiradenoma (GVES) which developed in 56-yr-old Korean woman. It is a rare variant of eccrine spiradenoma (ES), which might be mistaken for angiomatous lesions in view of its florid vascularity and hemorrhagic features. Histogenesis of GVES is not clearly elucidated although it is known that ES presumably originates in the eccrine glands. To clarify the histogenesis of GVES, immunohistochemical stainings using various monoclonal antibodies were also performed. The tumor was composed of three types of cells, namely pale epithelial cells, small basal cells, and myoepithelial cells. Therefore, we conclude that GVES originated from eccrine gland and mainly differentiates toward secretory portion of secretory coil.
Giant vascular eccrine spiradenoma (GVES) is a rare highly vascular variant of eccrine spiradenoma (ES), as only four cases are reported in the literature (1-3). Clinically, it might be mistaken for angiomatous lesions in view of its florid vascularity and hemorrhagic features. It is well known that tumor lobules are composed of two types of epithelial cells, namely small, dark staining basaloid cells located at the periphery and larger cells with a pale nucleus situated mostly in the center. However, cellular differentiation of GVES remains under discussion and the cells of which is composed are not clearly described. This tumor is considered to differentiate toward both the dermal duct and the secretory segment of the eccrine sweat gland like ES (4). This has been substantiated by the identification of occasional myoepithelial cells at the periphery of tubular structures (5). However, other authors have not found myoepithelial cells by ultrastuctural and histochemical studies. Furthermore, immunohistochemistry has failed to demonstrate myoepithelial differentiation (6). To clarify the nature of the cells of which is composed, we performed immunohistochemical stainings using various monoclonal antibodies for cytokeratins (CKs: CK, CK7, CK20, Cam5.2), epithelial membrane antigen (EMA), and carcinoembryonic antigen (CEA). Double-marker analysis was performed using p63 and smooth muscle actin (SMA) and triple-marker analysis was also performed using p63, SMA, and CK7.
A 56-yr-old healthy woman had a solitary violaceous protruding mass on her lower back. The tumor had begun as a small soft nodule approximately 3 yr before, and had grown slowly. The patient had no other complaint except paroxysmal pain. Physical examination showed a 2 cm sized, erythematous to violaceous hemispheric firm nodule on the left side of the lower back (Fig. 1). All laboratory findings were within normal limits. An excisional biopsy was performed with the clinical impression of a painful tumor of the skin such as angiolipoma or neuroma. There was no recurrence at the 12 months follow-up.
The resected tumor was routinely processed for light microscopy. For immunohistochemistry, formalin-fixed paraffin embedded tissue sections (4 to 5 m thick) were used. The antibody panel for epithelial cells included CK (Immunotech, Marseille, France), CK7 (ScyTec, Logan, UT, U.S.A.), CK20 (Biomeda, Foster City, CA, U.S.A.), Cam5.2 (Becton-Dickinson, San Jose, CA, U.S.A.), EMA (Signet Laboratories, Dedham, MA, U.S.A.), and CEA (Immunotech). The antibody panel for nerve fibers included neurofilament (Signet), and S-100 protein (Signet). For characterize myoepithelial cells, we used monoclonal antibodies for SMA (Immunotech) and p63 (clone 4A4, Oncogene Research Products, Boston, MA, U.S.A.). Recently, p63 has been proposed as a specific marker of precursor/stem cells (7) and ΔN-p63 is the predominant p63 isoform preferrentially expressed in the epithelial basal cells of organs such as the skin, breast, prostate and uterine cervix. It is also expressed in ductal structures of skin appendages and is also used as a marker of myoepithelial cells (8). Clone 4A4 is a mouse monoclonal antibody obtained from mice hyperimmunized with an aminoterminal fragment of the ΔN-p63 isoform expressed in E. coli as a GST fusion protein. Clone 4A4 also stained basal cells as well as myoepithelial cells.
Double marker analysis was performed using monoclonal antibody for p63 and SMA to differentiate basal cells and myoepithelial cells (8). Triple marker analysis using monoclonal antibody for p63, SMA, and CK7 was also performed to clarify three type of the cells ES is composed of, namely pale epithelial cells, small basal cells, and myoepithelial cells (9).
The cut surface of the gross specimen showed hemorrhagic nature in some parts. Microscopic examination revealed one large well-circumscribed encapsulated lobule and a small satellite lobule involving the dermis and subcutis. Unlike usual ES, a lobule consisted of peculiarly abundant stroma and compressed cellular cords or sheets that were composed of two types of cells: cells with large pale nuclei in the center and basaloid cells with small, dark nuclei at the periphery (Fig. 2, 3). The stroma showed greatly dilated vascular spaces containing pale pinkish lymph fluid and red blood cells (Fig. 2). Marked edematous or hyalinized perivascular stroma and a sprinkling of lymphocytes among tumor cells were characteristic histologic findings (Fig. 3).
The luminal large, pale epithelial cells were strongly positive for CK, CK7, Cam5.2, and EMA (Fig. 4) and negative for CK20. The outer layer of small basaloid cells were strongly positive for p63 and negative for SMA, CK, CK7, Cam5.2, and EMA. In addition, many p63+/SMA+ myoepithelial cells were present among tubules and sometimes around tubules (Fig. 5). Compressed cords of tumor cells were mostly composed of myoepithelial cells although there were scattered abortive tubules which were clearly recognized on CK, CK7, Cam5.2, and EMA immunostaining. CEA was only positive in luminal borders, luminal secretions, and intercellular canaliculi.
Based on the results of immunohistochemical findings, we concluded that the tumor was composed of pale epithelial cells, small basal cells, and myoepithelial cells. The tubules were composed of pale epithelial cells and small basal cells which were surrounded by the basement membrane with or without myoepithelial cells. These were clearly recognized on double- and triple-marker analysis (Fig. 5, 6).
S-100+/neurofilament+ compressed nerve fibers were present in the vicinity of the tumor lobules but not in the tumor lobules. Nerve fibers did not appear to be increased in number.
GVES, first described by Cotton et al. (1) in 1986, is a rare variant of ES. They report two cases of unusually large ES with marked degree of vascularity. Both were above 2 cm in size and histologically showed prominent blood-filled vascular spaces. This marked vascularity is an uncommon feature in sweat gland tumors and might suggest that this type of ES arise from a highly vascular region of the normal sweat gland (1). As far as we know, this is the fifth case of GVES in the literature. The clinical features of the previously reported cases of GVES are summarized in Table 1 (1-3). All cases of GVES, including our case, had made a faulty clinical diagnosis of angiolipoma, angiosarcoma, malignant melanoma, neuroma, sebaceous cyst, or venous thrombosis. It is emphasized that this rare type of ES may result in the erroneous diagnosis of angiomatous lesions by both clinicians and pathologists because of the florid vascularity and hemorrhagic features.
To clarify the histogenesis of GVES, we performed double- and triple-marker analysis. We found numerous p63+/SMA+ myoepithelial cells that were mostly arranged as compressing cellular cords or strands around edematous or hyalinized perivascular spaces or among tubules or ducts. Tubular structures were mostly composed of two types of epithelial cells, namely inner luminal (CK+/CK7+/Cam5.2+) and outer basal cells (p63+/SMA-) and had basement membrane. Some of the tubules were lined by myoepithelial cells. Normal intradermal and intraepidermal eccrine duct and ductal portion of eccrine secretory coil are lined by two types of epithelial cells, inner luminal cells (CK+/CK7-/Cam5.2-) and basal cells (p63+/SMA-) without myoepithelial cells and basement membrane. But secretory portion of eccrine secretory coil is composed of inner luminal cells (CK+/CK7+/Cam5.2+) and outer myoepithelial cells (p63+/SMA+) with basement membrane. Therefore, tubule or ducts seen in GVES did not strictly correspond to intradermal eccrine duct and ductal portion of secretory coil. Furthermore, findings of numerous myoepithelial cells and CEA+/EMA+ intercellular canaliculi support that GVES differentiates toward secretory portion of eccrine secretory coil (Table 2, 3).
Therefore, we conclude that GVES is a rare benign tumor originated from eccrine gland and mainly differentiates toward secretory portion of secretory coil.
Figures and Tables
Fig. 2
A large well-circumscribed encapsulated lobule in the dermis. The abundant stroma shows greatly dilated vascular spaces containing pale pinkish lymph fluid and red blood cells (H&E, ×20).

Fig. 3
Tubules are lined by two types of cells: cells with large pale nuclei and basaloid cells with small, dark nuclei. A sprinkling of lymphocytes among tumor cells are found (H&E, ×400).

Fig. 4
Immunohistochemical staining for CK (A), CK7 (B), Cam5.2 (C), and EMA (D). The luminal large, pale epithelial cells are strongly positive and the outer layer of small basaloid cells are negative (×400).
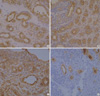
Fig. 5
Double marker analysis shows that nuclei of outer basal cells (arrow-head) of tubules are positive for p63 (brown color), and spindle shaped myoepithelial cells (arrow) are both positive for p63 (nucleus, brown color) and SMA (cytoplasm, reddish brown color) (×400).

Fig. 6
Triple marker analysis shows that tubules are lined by CK7+ inner luminal cells (arrow-head, reddish brown color) and p63+ basal cells (small-arrow, dark brown color), and there are many p63+/SMA+ myoepithelial cells (large-arrow, central nucleus is dark brown color and peripheral cytoplasm is blue color) (×400).

References
1. Cotton DW, Slater DN, Rooney N, Goepel JR, Mills PM. Giant vascular eccrine spiradenomas: a report of two cases with histology, immunohistology and electron microscopy. Histopathology. 1986. 10:1093–1099.

2. Hey A, Grouls V, Rockelein G. Vascular eccrine giant spiradenoma-a case report with histology and immunohistology of a rare variant of benign sweat gland tumors. Z Hautkr. 1988. 63:444–447.
3. Senol M, Ozcan A, Sasmaz S, Ozen S, Ciralik H. Giant vascular eccrine spiradenoma. Int J Dermatol. 1998. 37:221–223.

4. Watanabe S, Hirose M, Sato S, Takahashi H. Immunohistochemical analysis of cytokeratin expression in eccrine spiradenoma: similarities to the transitional portions between secretory segments and coiled ducts of eccrine glands. Br J Dermatol. 1994. 131:799–807.

5. Eckert F, Betke M, Schmoeckel C, Neuweiler J, Schmid U. Myoepithelial differentiation in benign sweat gland tumors. Demonstrated by a monoclonal antibody to alpha-smooth muscle actin. J Cutan Pathol. 1992. 19:294–301.

6. Maiorana A, Nigrisoli E, Papotti M. Immunohistochemical markers of sweat gland tumors. J Cutan Pathol. 1986. 13:187–196.

7. Tsujita-Kyutoku M, Kiuchi K, Danbara N, Yuri T, Senzaki H, Tsubura A. p63 expression in normal human epidermis and epidermal appendages and their tumors. J Cutan Pathol. 2003. 30:11–17.

8. Chilosi M, Zamo A, Brighenti A, Malpeli G, Montagna L, Piccoli P, Pedron S, Lestani M, Inghirami G, Scarpa A, Doglioni C, Menestrina F. Constitutive expression of DeltaN-p63alpha isoform in human thymus and thymic epithelial tumours. Virchows Arch. 2003. 443:175–183.
9. Krenacs T, Laszik Z, Dobo E. Application of immunogold-silver staining and immunoenzymatic methods in multiple labelling of human pancreatic Langerhans islet cells. Acta Histochem. 1989. 85:79–85.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download