Abstract
Here we report two cases of isolated diffuse mesangial sclerosis (IDMS) with early onset end-stage renal failure. These female patients did not show abnormalities of the gonads or external genitalia. Direct sequencing of WT1 PCR products from genomic DNA identified WT1 mutations in exons 8 (366 Arg>His) and 9 (396 Asp>Tyr). These mutations have been reported previously in association with Denys-Drash syndrome (DDS) with early onset renal failure. Therefore we suggest that, at least in part, IDMS is a variant of DDS and that investigations for the WT1 mutations should be performed in IDMS patients. In cases with identified WT1 mutations, the same attention to tumor development should be required as in DDS patients, and karyotyping and serial abdominal ultrasonograms to evaluate the gonads and kidney are warranted.
The Wilms' tumor suppressor gene (WT1), located at chromosome 11p13, has 10 exons, spans -50 kb, and encodes a zinc-finger-transcription factor presumed to regulate the expression of numerous target genes through DNA binding (1). WT1 was initially identified as a gene inactivated in Wilms' tumor, although the estimated percentage of Wilms' tumors (WTs) with WT1 mutations is only 10-15%. Investigations of WT1 have revealed the normal physiologic functions of this gene in embryogenesis, gonadogenesis and nephrogenesis (1). WT1 has been shown to play a role in kidney induction (2) and during later steps of nephrogenesis (3), and there are suggestions that WT1 may play an important role in the maintenance of normal podocyte function (4).
Besides Wilms' tumor, a number of human diseases have been shown to be associated with mutations of the WT1 gene (5). Genital abnormalities are noted in three of these disorders, WAGR syndrome (Wilms' tumor, aniridia, genitourinary malformation, and mental retardation), Denys-Drash syndrome (DDS), and Frasier syndrome (FS), and the analysis of WT1 knockout mice also suggested a fundamental role of WT1 in gonad development (6). Wilhelm and Englert reported that the WT1 regulates early gonad development by activation of steroidogenesis factor 1, Sf1, which encodes an orphan nuclear receptor that regulates the expression of several genes involved in steroidogenesis and gonadal development (7).
DDS traditionally encompasses patients with the triad of congenital nephrotic syndrome leading to end-stage renal failure (ESRF), XY pseudohermaphroditism and Wilms' tumor (8, 9). Subsequent reports described patients with incomplete forms of this syndrome (10-12), and the definition of DDS has now been widened to include XY individuals with mild to severe genital anomalies as well as XX individuals displaying nephropathy and WT (13). The nephropathies in DDS are characterized by the histological finding of diffuse mesangial sclerosis (DMS), a finding also described in some cases of congenital nephrotic syndrome (isolated diffuse mesangial sclerosis, IDMS). Analysis of genotype-phenotype correlation in IDMS suggests that at least some IDMS patients have a variant form of DDS due to WT1 mutations (14).
Attempts to correlate the phenotype of DDS and different WT1 mutations did not provide clear-cut results (15), and no correlation could be established between any particular mutation and the occurrence of Wilms' tumor. However, analysis of a large number of patients might provide clues to the role of each mutation, and might help in understanding the normal physiology of nephrogenesis and the prediction for tumorigenesis.
In this report, we present two cases of IDMS with different WT1 gene mutations that had previously been reported in association with DDS.
A 76-day-old girl was referred for evaluation of leg edema and albuminuria. She was born at 37+1 weeks' gestational age with a birth weight of 2,500 g. Placenta weight was 450 g. Physical examination on admission revealed generalized edema, ascites and normal female external genitalia. Laboratory findings showed; hemoglobin, 8.2 g/dL; cholesterol, 147 mg/dL; total serum protein, 3.5 g/dL; serum albumin, 2.1 g/dL; serum creatinine, 1.7 mg/dL; BUN, 27 mg/dL; total calcium, 5.6 mg/dL; phosphorus, 11.7 mg/dL; sodium, 116 mEq/L; potassium, 6.0 mEq/L; chloride 102 mEq/L; tCO2 5.7 mEq/L. Urinalysis revealed albuminuria and hematuria. She was anuric after admission, and peritoneal dialysis was commenced. Ultrasound examination revealed enlarged kidneys with increased parenchymal echogenicity. A renal biopsy was performed at the age of 111 days, and 30 glomeruli were examined. Light microscopy showed diffuse mesangial sclerosis (Fig. 1) and cortical tubular dilatation and microcyst formation. Subcapsular tubular atrophy and small immature glomeruli were also seen. Karyotype analysis showed 46 chromosomes, including XX. A WT1 mutation was identified by direct sequencing of a WT1 PCR product obtained from genomic DNA from white blood cells. Analysis of the WT1 exon 8 sequence revealed the presence of a heterozygous G to A base substitution, converting 366Arg to 366His (Fig. 2). This base substitution was absent in both parents. The patient is now 13 months old, and is well and on peritoneal dialysis, expecting renal transplantation. Abdominal ultrasonogram has revealed no mass lesions in the kidney or ovary.
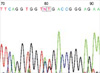
A 26-day-old girl was referred for evaluation of renal failure. She was born at 38+5 weeks' gestational age with a birth weight of 3,200 g. She was well until two days before admission when she developed generalized tonic clonic seizure. At a local clinic, hypocalcemia, hyperkalemia and azotemia were detected and she was referred to us. On admission, generalized edema was noted. Her external genitalia were normal female. Laboratory findings showed: hemoglobin, 9.8 g/dL; cholesterol, 120 mg/dL; total serum protein, 3.7 g/dL; serum albumin, 1.5 g/dL; BUN, 75 mg/dL; serum creatinine, 4.1 mg/dL; total calcium, 5.9 mg/dL; phosphorus, 12.7 mg/dL; sodium, 136 mEq/L; potassium, 5.5 mEq/L; chloride, 113 mEq/L; tCO2 7.8 mEq/L. Albuminuria and hematuria were detected. An ultrasonogram revealed enlarged kidneys with increased parenchymal echogenicity and poor corticomedullary differentiation. A renal biopsy was performed at the age of 29 days and light microscopy showed small glomeruli with various degrees of mesangial sclerosis (Fig. 3). The tubules were dilated with regenerative activity. Her karyotype was 46 XX. Analysis of the sequence of WT1 exon 9 revealed the presence of a heterozygous G to T base substitution, converting 396Asp to 396Tyr, and a heterozygous 395 Ser (TCC) > Ser (TCA) polymorphism (Fig. 4). Both parents showed normal sequence at these sites. Peritoneal dialysis was commenced with a temporary shift to hemodiafiltration because of dialysate leakage at the exit site. Sustained hypertension (120/80 mmHg) responded to an angiotensin-converting enzyme inhibitor. She was maintained on total parenteral nutrition due to uncontrolled chylothorax that resulted from internal jugular vein catheterization, and the patient died at the age of six months due to multiple serious infections and failure to thrive. Autopsy was not performed.
These two patients showed isolated diffuse mesangial sclerosis without genital abnormalities. The mutation present in the second patient was previously reported in a DDS patient with early onset ESRF resulting from DMS, ambiguous genitalia, and a 46 XY karyotype. Autopsy showed a streak gonad and nephroblastomatosis, but no WTs were revealed (16). As in our second patient, this boy reached ESRF within a few weeks. Considering that ESRF in DMS is reached within a few months or years (17), early onset ESRF in patients is noted as a feature of this mutation. The mutation in the first patient was also previously reported in a DDS patient (46 XY, female genitalia, streak gonad, DMS, gonadoblastoma, without WT) (18).
It is well known that WT1 has many roles during gonadogenesis, and since WT1 is expressed in the same cell lineage as SRY, the Y-located testis determinant, WT1 may be acting upstream to SRY during development of the genital ridge; perhaps controlling SRY expression, or interacting directly with SRY during sex determination, or functioning immediately downstream of SRY (19). WT1-null mutant mice of both sexes fail to develop kidneys and gonads, indicating that WT1 acts upstream of the sex-determining decision (6).
Frasier syndrome (FS) is another distinctive disease associated with WT1 mutation, characterized by male pseudohermaphroditism and nephropathy with late onset ESRF, and a frequent association with gonadoblastoma (20). Molecular analysis of a familial case of FS has been described, which provided a clue to WT1's effects on gonadogenesis. Two sisters in their late teens showed signs of renal disease and each sister has a donor splice-site mutation that was predicted to result in an imbalance of the KTS+/KTS- isoforms. One sister was 46 XY, with complete gonadal dysgenesis with normal female phenotype, while the other was 46 XX, with apparently normal ovarian development and function. This suggests that either WT1 has a male-specific function in sex determination or that testis formation is much more sensitive to dosage effects than ovarian formation (21).
Our two cases are female with karyotype 46 XX, which might be the reason for the absence of gonadal abnormalities, and we suspect that if a patient is born with karyotype 46 XY, there may be a possibility of DDS development. Although male patients have been described (14, 22), IDMS most often occurs in females; however, the percentage of patients with WT1 mutation-positive IDMS, the sex ratio and the associated risk of Wilms' tumor are still unknown. A recent study of seven Japanese IDMS patients reported a low rate of WT1 mutation (2/7) and a low risk of Wilms' tumor (0/7) (23). The mutations observed in these Japanese patients were different from those observed in the previous reports of DDS, and in the removed kidneys, nephrogenic nests were not found. Therefore, it was concluded that in IDMS with WT1 mutations, the risk of Wilms' tumor might not be very high. Our report is limited to two patients, but both mutations were already associated with DDS, and the early onset ESRF in patients with the 396 Asp (GAC) > Tyr (TAC) mutation is distinctively similar. This supports the concept that, at least in part, IDMS is a variant form of DDS, and we suggest that IDMS patients require the same attention as DDS patients because of the possible association with Wilms' tumor and/or gonadoblastoma.
Hu et al. (24) recently reported two boys with incomplete DDS with WT1 mutations who underwent prophylactic bilateral nephrectomy, and the removed kidneys showed nephroblastomatosis, which has malignant potential. Initial renal biopsy and imaging studies did not demonstrate these findings. The authors suggested that missense mutations in exons 8 and 9 of WT1 can be regarded as risk factors for development of Wilms' tumor and proposed prophylactic bilateral nephrectomy and early renal transplantation. It is still debatable whether prophylactic nephrectomies should be carried out in all children with DMS before renal transplantation, and Schumacher et al. (15) suggested that regular renal imaging might be necessary to evaluate the need for bilateral nephrectomy. Considering the clinical courses of the patients in the previous reports, our patients also might have had the possibility of malignant transformation, but we chose regular renal and gonadal imaging rather than prophylactic nephrectomy, and three-monthly ultrasonogram until death did not show evidence of malignancy in the second patient. The first patient is well on peritoneal dialysis with no sign of malignancy on three-monthly screening and we are considering of nephrectomy at the time of transplantation.
The reason for the heterogeneity of the clinical features of patients with WT1 mutations is not yet clear, and we suggest that a classification based on genetic diagnosis rather than syndromatic diagnosis might be more applicable for the prediction of outcome, and routine genetic analysis to detect WT1 mutation should be applied to all IDMS patients. If WT1 mutation is documented, karyotyping and serial abdominal ultrasonographies are warranted for the evaluation of tumor development in the gonad and kidney, and the analysis of genotype-phenotype correlations should be continued.
Figures and Tables
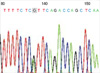
Fig. 2
Sequence analysis of WT1 exon 8 from the DNA of patient 1, with a G>A alteration changing amino acid 366 from Arg to His.
References
2. Dressler GR. Transcription factors in renal development: the WT1 and pax-2 story. Semin Nephrol. 1995. 15:263–271.
3. Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for WT1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999. 126:1845–1857.

4. Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet. 2002. 11:651–659.

5. Englert C. WT1; More than a transcription factor? Trends Biochem Sci. 1998. 23:389–393.
6. Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier , Houssman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993. 74:679–691.

7. Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002. 16:1839–1851.

8. Denys P, Malvaux P, van den Berghe H, Tanghe W, Proesmans W. De pseudohermaphrodisme masculin, d'une tumeur de Wilms, d'une nephropathie parenchymateuse et d'un mosaicisme XX/XY. Archives Francaises de Pediatrie. 1967. 24:729–739.
9. Drash A, Sherman F, Harmann WH, Blizzard RM. A syndrome of pseudohermaphroditism, Wilms' tumor, hypertension and degenerative renal disease. J Pediatr. 1970. 76:585–593.

10. Habib R, Loirat C, Gubler MC, Niaudet P, Bensman A, Levy M, Broyer M. The nephropathy associated with male pseudohermaphroditism and Wilms' tumor (Drash syndrome): a distinctive glomerular lesion? Report of 10 cases. Clin Nephrol. 1985. 24:269–278.
11. Jadresic L, Leake J, Gordon I, Dillon MJ, Grant DB, Pritchard J, Risdon RA, Barratt TM. Clinicopathologic review of twelve children with nephropathy, Wilms' tumor, and genital abnormalities (Drash syndrome). J Pediatr. 1990. 117:717–725.

12. Manivel JC, Sibley RK, Dehner LP. Complete and incomplete Drash syndrome: a clinicopathologic study of five cases of a dysontogenetic-neoplastic complex. Hum Pathol. 1987. 18:80–89.
13. Hastie ND. The genetics of Wilms' tumor-a case of disrupted development. Ann Rev Genet. 1994. 28:523–558.

14. Jeanpierre C, Denamur E, Henry I, Cabanis MO, Luce S, Cecille A, Elion J, Peuchmaur M, Loirat C, Niaudet P, Gubler MC, Junien C. Identification of constitutional WT1 mutations, in patients with isolated diffuse mesangial sclerosis and analysis of genotype/phenotype correlations by use of a computerized mutation database. Am J Hum Genet. 1998. 62:824–833.

15. Schumacher V, Schärer K, Wühl E, Altrogge H, Bonzel KE, Guschmann M, Neuhaus TJ, Pollastro RM, Kuwertz-Bröking E, Bulla M, Tondera AM, Mundel P, Helmchen U, Waldherr R, Weirich A, Royer-Pokora B. Spectrum of early onset nephrotic syndrome associated with WT1 missense mutations. Kidney Int. 1998. 53:1594–1600.

16. Little M, Carman G, Donaldson E. Novel WT1 exon 9 mutation (D396Y) in a patient with early onset Denys Drash syndrome. Hum Mutat. 2000. 15:389.

17. Holmberg C, Tryggvason K, Kestila MK, Jalanko HJ. Avner ED, Harmon WE, Niaudet P, editors. Congenital nephrotic syndrome. Pediatric Nephrology. 2004. 5th ed. Philadelphia: Lippincott Williams and Wilkins;503–516.
18. Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, Striegel JE, Houghton DC, Junien C, Habib R, Fouser L, Fine RN, Silverman BL, Haber DA, Housman D. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991. 67:437–447.

19. McElreavey K, Fellous M. Sex determination and the Y chromosome. Am J Med Genet. 1999. 89:176–185.

20. McTaggart SJ, Algar E, Chow CW, Powell HR, Jones CL. Clinical spectrum of Denys-Drash and Frasier syndrome. Pediatr Nephrol. 2001. 16:335–339.

21. Demmer L, Primack W, Loik V, Brown R, Therville N, McElreavey K. Frasier syndrome: a cause of focal segmental glomerulosclerosis in a 46,XX female. J Am Soc Nephrol. 1999. 10:2215–2218.
22. Ito S, Ikeda M, Takada A, Kikuchi H, Hata J, Honda M. Nephrotic syndrome and end-stage renal disease with WT1 mutation detected at 3 years. Pediatr Nephrol. 1999. 13:790–791.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download