Abstract
A 31-yr-old Korean woman was presented with 4-month history of bilateral hand swelling and stiffness. On clinical examination, she had a painful synovitis of both hands, wrists, knees and ankles. The radiologic and histological examinations confirmed it with palmar fasciitis and polyarthritis syndrome (PFPAS). PFPAS is an uncommon disorder characterized by progressive flexion contractures of both hands, inflammatory fasciitiis, fibrosis, and a generalized inflammatory arthritis. Although most reported cases of PFPAS have been associated with various malignancies, our patient have not been associated with malignancy during 24 months follow up period from her first symptom onset. Her symptoms were improved with moderate dose of corticosteroid and she is currently taking prednisone 5 mg daily without any evidence for internal malignancy. We present here in a young Korean patient with idiopathic PFPAS who was successfully treated with administration of corticosteroid.
Palmar fasciitis and polyarthritis syndrome (PFPAS) is an uncommon disorder characterized by progressive flexion contractures of both hands, inflammatory fasciitis, fibrosis, and a generalized inflammatory arthritis (1, 2). The course might be rapidly progressive and result to a loss of function of the upper extremity due to contractures and pain. In 1982, Medsger et al. (1) described PFPAS as a disease entity, which should be differentiated from reflex sympathetic dystrophy (RSD) and Dupuytren's contracture. More than 40 cases have since been reported, but the exact etiology of PFPAS is still not known. Most of them have been suggested associations with various malignancies (3, 4) and only a few reports were not associated with malignancy (5, 6).
We present herein a young woman with PFPAS in the absence of an associated internal malignancy.
A 31-yr-old Korean woman presented in Febraury, 2004, with 4-month history of bilateral hand swelling and stiffness. This condition began suddenly with pain and decreased ability to hold objects. A trial of non-steroidal anti-inflammatory drug was not effective. She had been healthy and her past medical history was not significant except intermittent arthralgia that had begun 1 yr before visiting our clinic. She denied any rheumatic disorder, Raynaud's phenomenon, or trauma.
On clinical examination, she had a painful synovitis of both hands, wrists, knees and ankles. Her hands were mildly edematous (Fig. 1). However, the overlying skin was normal, with no evidence of telangiectasia, digital pitting, or of systemic sclerosis elsewhere. She was unable to flex or extend her fingers. The hands were warm with an intact sensory examination and normal sweat pattern. The radiography of both hands showed soft tissue swellings on PIP joints without erosive changes and other joint radiographs were normal. Technetium-99m bone scan showed a slight increased uptake in both hands, wrists, shoulders, knees, ankles, and feet. A magnetic resonance image (MRI) of the forearm revealed diffuse enhancements in the fascia, extensor and flexor tendons and some muscles including the extensor digiti minimi muscle and flexor carpi ulnaris muscles (Fig. 2).
Laboratory studies revealed normal erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and routine chemistry results as well as a normal complete blood count (CBC) without eosinophilia. Thyroid hormone and TSH were each in normal ranges. Antinuclear antibody was positive at a titer of 1:80, while the other antibodies were negative.
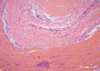
A full-thickness skin biopsy was obtained from the dorsal surface of the left forearm showing abnormal intensity on MRI because an incision of the palmar fascia was thought to result in impairment of hand function. The biopsy demonstrated that the fascia was thickened and fibrotic with perivascular infiltration of mononuclear cells. Neither eosinophil nor granulocyte infiltration was found in any specimen of fascia (Fig. 3). The underlying skeletal muscle showed well preserved myocytes, mild interstitial edema, and fibrosis. Marked interstitial infiltration, mainly of T lymphocytes and focal infiltration of B lymphocytes with scattered plasma cells, were observed on immunohistochemical stainings. There was no evidence of perifascicular atrophy, necrosis, granuloma or leukocytoclastic vasculitis.
Because of her unusual clinical features, she was screened for malignant disease. We performed endoscopic and radiologic examinations for internal organ to find out the presence of malignancy but those were negative. Examinations for the breast, thyroid, and pelvis showed no abnormal findings. Tumor markers including CA125, CA19-9, AFP, and CEA were each in normal ranges.
The patient started on prednisone 30 mg daily. Her symptoms such as arthralgia and bilateral hand swelling with stiffness had gradually improved over 2 months and the dosage of prednisone was tapered gradually. MRI study which performed 6 months after taking medication revealed no inflammatory reactions in both fascia and muscles of the forearm. Follow up bone scan also showed no increased uptake in all the joints. Twenty-four months after her first symptom onset, she is currently taking prednisone 5 mg daily without evidence for internal malignancy in radiological and serologic tests.
Palmar fasciitis is a rare condition in which the patient develops rapidly progressive contractures of both hands and arthritis of the wrists and larger joints. The underlying immunological mechanisms have not been defined so far and may include the activation of certain factors with profibrotic activities, transforming growth factor β or connective tissue growth factor (7). Medsger et al. (1) suggested that a fibroblast proliferative factor might be secreted by the tumor. They were unsuccessful, however, in their attempt to show a proliferative factor in the laboratory. The majority of affected patients have been women, which suggests that the female hormonal state may predispose to this syndrome as is the case in most autoimmune diseases.
Most of reported cases have been suggested associations with neoplasms, most commonly in elderly patients, preceding or accompanying the diagnosis of carcinoma of the ovary. They followed by neoplasms of the breast, pancreas, lung, stomach, and endometrium. Table 1 summarizes the published cases of PFPAS associated with malignancies. The rheumatic symptoms may precede detection of the tumor by 1-23 months. They have confirmed improvement in rheumatic symptoms after chemotherapy but it is unclear whether this is a result of tumor necrosis, immunomodulation, or anti-inflammatory effects.
Some PFPAS patients without apparent neoplasms have been described in previous reports. Seaman et al. (6) reported seven patients undergoing antituberculosis therapy, who developed palmar fasciitis within 4 months of the addition of the drug ethionamide and the fasciitis resolved when ethionamide was discontinued. Until now, only one idiopathic PFPAS of 75-yr-old white woman was reported by Laszlo et al. (5) in 1995. However, they could not exclude the possibilities of future developing of any malignancy because of their short duration (12 months) of follow up. Because of this well documented association between palmar fasciitis, arthritis, and neoplasms, we performed a full work up for her to detect a hidden malignancy. It has been negative both at the time of diagnosis and until now. We regard the present case as idiopathic because we have been followed her up more than 24 months after her first symptom without any evidence of internal malignancy. The patient was improved with high dose of corticosteroid, while most PFPAS patients associated with malignancy did not responsive to steroid treatment.
The differential diagnosis includes other conditions associated with contractures of the hands. The musculoskeletal manifestations of PFPAS are similar to RSD but is characterized by its considerably more aggressive nature and diffuse articular involvements. Most cases of RSD have a preceding noxious event and symptoms of vasomotor disturbances. Bone scanning of the patient with RSD usually shows an asymmetry between affected and non-affected limbs. Our patient, however, did not show those clinical features. In addition, the pathological finding of our patient was far from that of RSD characterized by muscle changes consisting of type I fiber atrophy, an increase in lipofuscin and capillary abnormalities with thickening of the basement membrane (31).
Dupuytren's contracture is a relatively common condition characterized by nodular thickening and contraction of the palmar fascia. Its manifestation is quite similar with that of PFPAS, but it usually affects the ulnar side of both hands and it is more common in aged man. The findings of our patient differed from the disorder in that the fasciitis was extending to the forearms and apparent polyarthritis was present. Pathologically, the early lesion of Dupuytren's disease is characterized by marked fibroblastic proliferation, vascular hyperplasia and clusters of macrophages and T lymphocytes. This is followed by dense, disorderly collagen deposition with thickening of the palmar fascia and nodule formation (32). These are different from the pathological findings of PFPAS.
Eosinophilic fasciitis is characterized by painful and erythematous swelling of the extremities, accompanied by rapid weight gain, fever and myalgia. Eosinophilia in the peripheral blood, and somewhat less commonly in affected tissue, is prominent at the acute stage (33). In this disorder, however, the hands are usually spared. Our patient had no eosinophilia in peripheral blood or eosinophilic infiltration in the tissue.
In summary, we have presented a young Korean patient with idiopathic PFPAS who was successfully treated with administration of corticosteroid. Therefore, we suggest that close search for underlying malignancy and follow ups are essential to PFPAS patient but in a case not associated with malignancy like our case, administration of steroid might be an effective option for treatment.
Figures and Tables
Fig. 1
Hands of the patient. It shows the digital flexion contractures and the thickened palmar fascia. The MCP, and PIP joints were tender, and swollen.

Fig. 2
T1WI gadolinium enhanced MRI of the left forearm. It shows diffuse enhancements in the fascia, extensor and flexor tendons and some muscles.
Fig. 3
Biopsy finding. The fascia is thickened and fibrotic with perivascular infiltration of mononuclear cells. The underlying skeletal muscle shows well preserved myocytes, mild interstitial edema and marked interstitial infiltration mainly of lymphocytes. There is no evidence of perifascicular atrophy, necrosis, granuloma or leukocytoclastic vasculitis (H&E stain ×40).
References
1. Medsger TA, Dixon JA, Garwood VF. Palmar fasciitis and polyarthritis associated with ovarian carcinoma. Ann Intern Med. 1982. 96:424–431.

3. Pfinsgraff J, Buckingham RB, Killian PJ, Keister SR, Brereton WF, Weinblatt ME, George DL, Arnett FC. Palmar fasciitis and arthritis with malignant neoplasms: a paraneoplastic syndrome. Semin Arthritis Rheum. 1986. 16:118–125.

4. Enomoto M, Takemura H, Suzuki M, Yuhara T, Akama T, Yamane K, Sumida T. Palmar fasciitis and polyarthritis associated with gastric carcinoma: complete resolution after total gastrectomy. Intern Med. 2000. 39:754–757.

6. Seaman JM, Goble M, Madsen L, Steigerwald JC. Fasciitis and polyarthritis during antituberculous therapy. Arthritis Rheum. 1985. 28:1179–1184.

7. Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002. 14:681–685.
8. Baer AN, Phillips RM Jr. Pancreatic carcinoma and palmar fasciitis. Ann Intern Med. 1983. 99:411–412.

9. Shiel WC Jr, Prete PE, Jason M, Andrews BS. Palmar fasciitis and arthritis with ovarian and non-ovarian carcinomas. New syndrome. Am J Med. 1985. 79:640–644.

10. Valverde-Garcia J, Juanola-Roura X, Ruiz-Martin JM, Nolla-Sole JM, Rodriguez-Moreno J, Roig-Escofet D. Paraneoplastic palmar fasciitis-polyarthritis syndrome associated with breast cancer. J Rheumatol. 1987. 14:1207–1209.
11. Caron P, Lassoued S, Thibaut I, Fournie B, Fournie A. Thyroid plasmacytoma with dermatomyositis and palmar fasciitis. J Rheumatol. 1989. 16:997–999.
12. Willemse PH, Mulder NH, van de Tempel HJ, Aalders JG, Sleijfer DT. Palmar fasciitis and arthritis in a patient with an extraovarian adenocarcinoma of the coelomic epithelium. Ann Rheum Dis. 1991. 50:53–54.

13. Van den Bergh L, Vanneste SB, Knockaert DC. Palmar fasciitis and arthritis associated with cancer of the prostate. Acta Clin Belg. 1991. 46:106–110.
14. Strobel ES, Lacour M, Peter HH. Palmar fascial thickening and contractures of fingers resembling arthritis-a paraneoplastic symptom? Rheumatol Int. 1992. 12:79–80.
15. Leslie BM. Palmar fasciitis and polyarthritis associated with a malignant neoplasm: a paraneoplastic syndrome. Orthopedics. 1992. 15:1436–1439.

16. Vinker S, Dgani R, Lifschitz-Mercer B, Sthoeger ZM, Green L. Palmar fasciitis and polyarthritis associated with ovarian carcinoma in a young patient. A case report and review of the literature. Clin Rheumatol. 1996. 15:495–497.

17. Dhote R, Beuzeboc P, Permal S, Poiraudeau S, Revel M, Christoforov B. Paraneoplastic palmar fasciitis and achalasia-like esophageal disorder in a patient with breast cancer. Rev Rhum Engl Ed. 1997. 64:350–352.
18. Saxman SB, Seitz D. Breast cancer associated with palmar fasciitis and arthritis. J Clin Oncol. 1997. 15:3515–3516.

19. Wright GD, Doherty M. Unusual and memorable. Paraneoplastic palmar fasciitis and arthritis syndrome. Ann Rheum Dis. 1997. 56:626.
20. Grados F, Houvenagel E, Cayrolle G, Bellony R, Fardellone P, Sebert JL. Two new cancer locations accompanied with palmar fasciitis and polyarthritis. Rev Rhum Engl Ed. 1998. 65:212–214.
21. Eekhoff EM, van der Lubbe PA, Breedveld FC. Flexion contractures associated with a malignant neoplasm: 'A paraneoplastic syndrome?'. Clin Rheumatol. 1998. 17:157–159.

22. Matteson EL. Groove sign in paraneoplastic palmar fasciitis. J Rheumatol. 1998. 25:2043–2045.
23. Kase H, Aoki Y, Sugaya S, Takakuwa K, Tanaka K. Palmar fasciitis and polyarthritis associated with squamous cell carcinoma of the cervix. Int J Gynecol Cancer. 2000. 10:507–509.

24. Roman S, Tebib J, Scoazec JY, Menard Y, Paliard P, Dumortier J. Palmar fasciitis and paraneoplastic polyarthritis associated with hepatocellular carcinoma. Gastroenterol Clin Biol. 2001. 25:203–204.
25. Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002. 21:63–65.

26. Virik K, Lynch KP, Harper P. Gastroesophageal cancer, palmar fasciitis and a matrix metalloproteinase inhibitor. Intern Med J. 2002. 32:50–51.

27. Rammeh N, Elleuch M, Meddeb N, Sahli H, Cheour E, Sellami S. Palmar-plantar fasciitis and multiple myeloma. Tunis Med. 2003. 81:889–893.
28. Martorell EA, Murray PM, Peterson JJ, Menke DM, Calamia KT. Palmar fasciitis and arthritis syndrome associated with metastatic ovarian carcinoma: a report of four cases. J Hand Surg. 2004. 29:654–660.

29. Denschlag D, Riener E, Vaith P, Tempfer C, Keck C. Palmar fasciitis and polyarthritis as a paraneoplastic syndrome associated with tubal carcinoma: a case report. Ann Rheum Dis. 2004. 63:1177–1178.

30. Giannakopoulos ChK, Kyriakidou GK, Toufexi GE. Palmar fasciitis and polyarthritis associated with secondary ovarian carcinoma. Case report. Eur J Gynaecol Oncol. 2005. 26:339–341.
31. Kozin F. Reflex sympathetic dystrophy syndrome: a review. Clin Exp Rheumatol. 1992. 10:401–409.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download