Abstract
Scleroderma (SSc) renal crisis has been reported to be associated with anti-RNA polymerase I and III (RNAP I/III) antibodies in Caucasians and the Japanese. However, no report is available for Korean SSc patients. Here, we describe the case of a 65-yr-old female SSc patient who developed renal crisis and whose serum contained anti-RNAP I/III antibodies. She was finally diagnosed as having diffuse cutaneous SSc based on skin thickening proximal to the elbows and knees. Sudden hypertension, oliguria, and pulmonary edema were features of her renal crisis. Despite the use of captopril and adequate blood pressure control, her renal function deteriorated. Subsequent renal biopsy findings showed severe fibrinoid necrosis with luminal obliteration in interlobar arteries and arterioles consistent with SSc renal crisis. Serum anti-RNAP I/III antibodies were detected by radioimmunoprecipitation. This is the first report of a renal crisis in a Korean SSc patient with RNAP I/III antibodies.
Systemic sclerosis (SSc, scleroderma) is an autoimmune disease characterized by the accelerated accumulation of extracellular matrix and by various immunologic abnormalities (1). Serum autoantibodies are helpful markers because they have been correlated with certain clinical features of SSc (2, 3). Of the various SSc-related antibodies, anti-RNA polymerase (RNAP) antibodies are known to be SSc specific and to be present in 4-33% of SSc patients (4-8). There are three classes of RNAPs (RNAPs I, II, and III) (9) and anti-RNAP I and III (I/III) antibodies have been detected exclusively in SSc patients; moreover, the presence of these antibodies is known to be associated with diffuse cutaneous involvement and renal crisis (5, 6, 10, 11). Although the association between scleroderma renal crisis and anti-RNAP I/III antibodies has been reported in Caucasians and in the Japanese, no such report has been issued in the Korean SSc population. Furthermore, autoantibodies in Korean SSc patients show several distinctive features; 1) no association between disease subset and autoantibodies, such as, anti-topoisomerase I (anti-topo I) or anticentromere antibody (ACA), 2) a much lower prevalence of ACA in a limited subset (6.7-8.0% vs. 44% in Caucasians and 37% in the Japanese), and 3) no significant difference in the clinical characteristics of disease subsets, except for more frequent musculoskeletal involvement in a limited subset (12-14). Here, we report for the first time a case of renal crisis in a Korean SSc patient with serum anti-RNAP I/III antibodies detected by radioimmunoprecipitation (5).
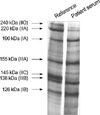
A 65-yr-old female visited the Rheumatology Clinic due to the thickening of hand and facial skin with digital pallor and cyanosis on cold exposure, which had developed 2 months previously. On the first visit to the clinic, her blood pressure was 130/80 mmHg and physical examination revealed skin thickening on the fingers of both hands, and on the right hand dorsum and right forearm. She was diagnosed as having systemic sclerosis of the limited cutaneous subset based on the American Rheumatism Association preliminary criteria (15). Laboratory data showed white blood cells at 8.39×109/L, hemoglobin 12.1 g/dL, platelets 240×109/L, ESR 8 mm/hr (normal range: 0-20), GOT 21U/L, GPT 18 U/L, total bilirubin 0.6 mg/dL, albumin 3.7 g/dL, BUN 11 mg/dL, and creatinine 0.7 mg/dL. Antinuclear antibody was positive, but ACA and anti-topo I were negative. Pulmonary function testing produced the following; forced vital capacity, 73%; forced expiratory volume in 1 sec, 79%; and diffusing capacity of carbon monoxide over volume of alveoli, 121%. High resolution computed tomography of the lungs revealed old tuberculosis in the right lower lobe with pleural thickening and calcifications without evidence of interstitial lung disease. Prazosin 1 mg/day was administered for Raynaud's phenomenon and intermittent antihistamines for skin pruritus. Twenty-two months after the diagnosis of limited SSc, her skin thickening began to rapidly progress above elbows and knees, and finally involved the trunk. Blood pressure was in the normal range. The diagnosis was converted to SSc of the diffuse cutaneous subset and she was started on D-penicillamine 250 mg/day. A month later, she visited the emergency room due to the sudden onset of facial edema and severe dyspnea. Her blood pressure was 220/134 mmHg, heart rate 120 beats/min, respiration rate 48/min, and body temperature 35℃. She reported that the dyspnea had begun 10 days previously and that her urine output had decreased markedly. A physical examination revealed facial and pre-tibial edema, and pulmonary rales in the whole lung field. Laboratory data showed hemoglobin at 9.9 g/dL, LDH 703 IU/L, total bilirubin 2.9 mg/dL, indirect bilirubin 1.8 mg/dL, and schistocytes with polychromasia on peripheral blood smear, suggesting intravascular hemolysis. Antineutrophil cytoplasmic antibody was negative. Urinalysis and microscopic examination showed mild albuminuria (1+by dipstick test), hematuria (≥100 red blood cells per high power field with dysmorphic red blood cells >90%), and pyuria (20-29 white blood cells per high power field). Urine culture grew no organisms. Azotemia was also detected with a BUN of 31 mg/dL and a creatinine of 2.2 mg/dL. Her chest radiograph showed cardiomegaly and bilateral hilar infiltrates consistent with pulmonary edema. She was transferred to intensive care unit, intubated, and assisted with a mechanical ventilator. Under a diagnosis of scleroderma renal crisis, captopril was begun, but failed to reverse a deteriorating renal function despite successful blood pressure control within 2 days. Renal biopsy showed severe fibrinoid necrosis with luminal obliteration in interlobar arteries and arterioles, strongly suggesting scleroderma renal crisis (Fig. 1). Immunofluorescence staining showed no evidence of immune complex or autoantibody deposition. Because scleroderma renal crisis has been reported to be associated with anti-RNAP I or III antibodies, we tested whether this patient had anti-RNAP antibodies in her serum. Indeed, anti-RNAP I/III antibodies were detected by radioimmunoprecipitation using 35S-methionine-labeled K 562 cells (Fig. 2). Hemodialysis was started and has been continued for 3 yr without renal recovery.
This is the first case report of scleroderma renal crisis with the presence of RNAP I/III antibodies in the Korean population. Renal crisis in this patient was diagnosed by accelerated hypertension and acute renal failure with a typical renal pathology. The presence of serum anti-RNAP antibodies was detected by radioimmunoprecipitation. The clinical findings in association with the presence of anti-RNAP I/III antibodies in this case are consistent with previous reports of SSc patients (5, 6, 10, 11, 16, 17), with respect to; 1) renal crisis in association with anti-RNAP I/III antibodies, 2) the presence of anti-RNAP I/III antibodies in a diffuse subset patient, and 3) the absence of anti-topo I in the presence of anti-RNAP I/III antibodies.
Although not all SSc patients with anti-RNAP I/III antibodies develop renal crisis, renal crisis occurs in anti-RNAP I/III antibody positive patients much more frequently than in negative patients (5, 6, 11). Moreover, it is well known that the early administration of angiotensin converting enzyme (ACE) inhibitors can improve renal outcome. Therefore, knowing whether anti-RNAP I/III antibodies are present before renal crisis onset should alert physicians to the possibility of renal crisis and facilitate the initiation of prompt treatment.
According to Steen et al. (18), 44% (24/55) of scleroderma renal crisis patients who received ACE inhibitors, either died or required permanent dialysis while 56% (31/55) of them did not require dialysis at all or needed only transient dialysis. The factors that were associated with poor renal outcome were old age (>55 yr) (odds ratio, 12.28; p=0.002) and the presence of congestive heart failure (odds ratio, 3.89; p=0.03). This patient showed both factors and administration of captopril failed to improve her renal function. The poor response to ACE inhibitor in this patient suggests that her renal function had irreversibly deteriorated prior to initiation of medical treatment because she had symptoms of acute renal failure for at least 10 days before she was treated.
RNAPs (RNAPs I, II, and III) are multimeric proteins that are composed of 8-14 subunits of 10 to 220 kDa (9). The two largest subunits are unique to each class and are readily distinguishable by their characteristic mobilities on SDS-polyacrylamide gel (6, 7). Because of the size and complexity of RNAPs, it has proven difficult to develop methods for detecting anti-RNAP antibodies that are applicable in a clinical laboratory setting despite the potential benefit of knowledge on the presence of anti-RNAP I/III antibodies. However, recently, it was reported that anti-RNAP antibodies can be detected reliably using an enzyme-linked immunosorbent assay method (17, 19), which might help measure these antibodies routinely in the near future.
In summary, we report a case of renal crisis in the presence of anti-RNAP I/III antibodies in a Korean SSc patient of a diffuse cutaneous subset. Further studies with a sufficient number of patients are needed to confirm the association between the presence of anti-RNAP I/III antibodies and clinical features in the Korean population.
Figures and Tables
References
1. Seibold JR. Ruddy S, Harris ED, Sledge CB, editors. Scleroderma. Kelley's textbook of rheumatology. 2001. 12th edition. Philadelphia: WB Saunders;1211–1240.
2. Tan EM, Rodnan GP, Garcia I, Moroi Y, Fritzler MJ, Peebles C. Diversity of antinuclear antibodies in progressive systemic sclerosis: anticentromere antibody and its relationship to CREST syndrome. Arthritis Rheum. 1980. 23:617–625.
3. Van Venrooij WJ, Stapel SO, Houben H, Habets WJ, Kallenberg CG, Penner E, Van de Putte LB. Scl-86, marker antigen for diffuse scleroderma. J Clin Invest. 1985. 75:1053–1060.
4. Reimer G, Rose KM, Scheer U, Tan EM. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987. 79:65–72.

5. Kuwana M, Kaburaki T, Mimori T, Tojo T, Homma M. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest. 1993. 91:1399–1404.

6. Okano Y, Steen VD, Medsger TA Jr. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med. 1993. 119:1005–1013.

7. Hirakata M, Okano Y, Pati U, Suwa A, Medsger TA Jr, Hardin JA, Craft J. Identification of autoantibodies to RNA polymerase II: occurrence in systemic sclerosis and association with autoantibodies to RNA polymerase I and III. J Clin Invest. 1993. 91:2665–2672.
8. Satoh M, Ajmani AK, Ogasawara T, Langdon JJ, Hirakata M, Wang J, Reeves WH. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. J Clin Invest. 1994. 94:1981–1989.
9. Sentenac A. Eukaryotic RNA polymerases. CRC Crit Rev Biochem. 1985. 18:31–90.
10. Reimer G, Steen VD, Penning CA, Medsger TA Jr, Tan EM. Correlates between autoantibodies to nucleolar antigens and clinical features in patients with systemic sclerosis (scleroderma). Arthritis Rheum. 1988. 31:525–532.

11. Bardoni A, Rossi P, Salvini R, Bobbio-Pallavicini F, Caporali R, Montecucco C. Autoantibodies to RNA-polymerases in Italian patients with systemic sclerosis. Clin Exp Rheumatol. 2003. 21:301–306.
12. Kuwana M, Okano Y, Kaburaki J, Tojo T, Medsger TA Jr. Racial differences in the distribution of systemic sclerosis-related serum antinuclear antibodies. Arthritis Rheum. 1994. 37:902–906.

13. Kang SH, Park MH, Song EY, Kang SJ, Lee EB, Song YW, Takeuchi F. Association of HLA class II genes with systemic sclerosis in Koreans. J Rheumatol. 2001. 28:1577–1583.
14. Kang SW, Lee YJ, Cha HS, Kim HA, Park MH, Oh MD, Song YW, Choi KW, Lee EB, Han CW, Baek HJ. Study on the clinical characteristics of systemic sclerosis. Korean J Med. 1999. 57:979–987.
15. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980. 23:581–590.
16. Harvey GR, Butts S, Rands AL, Patel Y, McHugh NJ. Clinical and serological associations with anti-RNA polymerase antibodies in systemic sclerosis. Clin Exp Immunol. 1999. 117:395–402.

17. Chang M, Wang RJ, Yangco DT, Sharp GC, Komatireddy GR, Hoffman RW. Analysis of autoantibodies against RNA polymerases using immunoaffinity-purified RNA polymerase I, II, and III antigen in an enzyme-linked immunosorbent assay. Clin Immunol Immunopathol. 1998. 89:71–78.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download