Abstract
We present two patients who had acute paraplegia with sensory loss due to spontaneous spinal epidural hematoma (SSEH). One had myocardial infraction and the other had deep vein thrombosis, and the former was treated with anticoagulants and the latter was treated with thrombolytic agent. We analyzed the neurological status of our two cases each between its preoperative and postoperative state. Postoperatively both showed no improvement of neurologic symptom, and on follow-up of 12 months, one showed no neurologic improvement and the other showed a insignificant improvement of lower extremity muscle power (trace knee extensor/ankle dorsiflexor). We thought that this poor outcome was due to delayed operation, which was done more than 24 hr after the symptom onset. The outcome in SSEH is essentially determined by the time taken from symptom onset to operation. Therefore, early and precise diagnosis such as careful history taking and MRI evaluation is necessary.
Spontaneous spinal epidural hematoma (SSEH) represents 0.3-0.9% of spinal epidural space-occupying lesions. The clinical course has characteristics: without preceding trauma, patients experience an acute onset of local discomfort or pain, sometimes with radicular paresthesia. Within hours, signs of spinal cord compression appear, presenting as progressive paraplegia and loss of sensory function. Despite the clinical syndrome of SSEH is relatively characterized, its exact diagnosis may be difficult. It is important to recognize these signs since early diagnosis and prompt surgical evacuation provide the maximum chance of functional recovery.
A 51-yr-old man with a history of myocardial infarction was treated with percutaneous transluminal coronary angioplasty. After being discharged, he visited the department of internal medicine for three months and was taking 200 mg/day of Aspirin. The patient felt the discomfort of midback area and mild lower extremity weakness 11 days before admitted to emergency room (ER). He ignored these discomforts which was not a great deal in his daily activity and on the 11th day from the onset of symptoms, he swam as usual in a pool for about 1 hr. After swimming, he felt worsening back pain and aggravated lower extremity weakness and so he visited ER and was examined by a physician in ER. His physician noticed the rapidly progressive paraplegia and a loss of leg sensory function, and consulted the department of neurology. Neurologic physician who suspected the acute spinal cord disorder, ordered high dose steroid injection and serial neurologic examination. The neurologist thought this patient's symptom was not a surgical condition but a medical condition. The following day neurologist reexamined the neurologic status. The patient's neurologic status showed complete paraplegia in the lower extremity, T10-T12 dermatome hypesthesia, L1 below dematome anesthesia, no bladder function, patella tendon reflex (-/-), and ankle jerk (-/-). His coagulation profile showed PT: 0.85 (0.9-1.10 INR), aPTT: 40.2 (29.8-41.8 sec). The neurologist ordered MRI. MRI scan showed an epidural hematoma with spinal cord compression from T8 to L2 (Fig. 1, 2). Then the neurologist consulted department of orthopedic surgery. It was 28 hr after symptom onset, we performed the bilateral decompressive laminectomy from T 8 to L2 immediately. A large posterolateral hematoma and old blood clot was found within the epidural space (Fig. 3). No neurologic improvement was noted after surgery. On follow-up 21 months, his neurologic status showed a complete paraplegia, L1 below anesthesia, no bladder function and no deep tendon reflex.
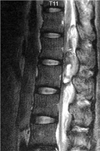
A 39-yr-old man was diagnosed with deep vein thrombosis (left common iliac, external iliac, superficial femoral, common femoral vein) at department of general surgery, and he was treated with the infusion catheter urokinase (1,100,000 unit/14 hr). The following day, he was performed with aspiration thrombectomy and stent at the department of diagnostic radiology, followed by the infusion catheter urokinase (1,000,000 unit/6 hr) treatment. During that evening, at 6:00 p.m., he felt the lower back discomfort and mild pain. And the physician ordered a pain killer. Because this pain disappeared soon, the physician was not in an account. The morning after onset of symptom, he felt decreased motor and sensory loss. His physician ordered MRI. MRI scan showed an epidural hematoma with spinal cord compression from T11 to L2 (Fig. 4, 5). His physician consulted department of orthopedic surgery. At 24 hr after symptom onset, we examined the neurologic status. The patient's neurologic status showed a complete motor loss except a right iliopsoas muscle power trace, L1-2 dermatome hypesthesia, L3 below dermatome anesthesia, perianal sense (-), patella tendon reflex (-/-), and ankle jerk (-/-). His coagulation profiles showed PT: 1.3 (0.9-1.10 INR), aPTT: 34.8 (28-40 sec). We performed the bilateral decompressive laminectomy from T 12 to L2 immediately. A large posterolateral hematoma was found within the epidural space (Fig. 6). Postoperative MRI shows extended dural sac and cord but no neurologic improvement was noted after surgery. On follow-up 12 months, his neurologic status showed a complete motor loss except the right iliopsoas, quadriceps, tibialis anterior muscle power trace, no sensory recovery, no bladder function, and no deep tendon reflex.
Spontaneous spinal epidural hematoma occurs infrequently. Approximately 350 cases have been reported in the medical literature, and the annual incidence has been estimated at 0.1 per 100,000. Predisposing factors such as inherited coagulopathy, spinal vascular malformation, anticoagulant therapy, therapeutic thrombolysis, and epidural analgesia, have been implicated, along with hypertension and antiplatelet therapy (1, 2). The clinical course is characteristic: without preceding trauma, patients experience an acute onset of local discomfort or pain, sometimes with radicular paresthesia. Within hours, signs of spinal cord compression appeared, and paraplegia and loss of sensory function progress. Despite the clinical syndrome of SSEH is relatively characteristic, its exact diagnosis may be difficult. Differential diagnosis includes spinal abscess, tumor, ischemia, transverse myelitis or acute vertebral disc disease (3).
Because the results of operative decompression of the spinal cord mainly depend on the duration of the symptoms, the time lost for diagnostic measures may have negative influence on the functional outcome. Therefore, immediate neuroradiologic confirmation of the clinical diagnosis is necessary. Currently, MRI has been used as the first choice of diagnostic method for spinal emergencies because it allows rapid evaluation of large parts of the vertebral column and the spinal cord.
The prognosis of neurologic deficits predominantly depends on the time interval between the onset of symptoms and the surgical decompression (2-6). In our patients, surgery was delayed because patient and physician made light of symptom. So the preoperative neurologic status was aggravated, and unfortunately as a result no improvement of symptom showed. Both the patient and the doctor should not take early, but mild, signs lightly which may result in delayed diagnosis, like our two cases. In SSEH, the small signs such as minor pain and slight discomfort appear, followed by rapid neurological disorder, thus, these small signs should be considered prudently and immediately carry out MRI to make quick and accurate diagnosis before the patient's neurological state is aggravated. The prognosis appears to be related to the severity of the preoperative neurologic deficit and early operation for rapid decompression of the spinal cord is crucial. Lawton et al. (7) confirmed the relationship between neurologic recovery, the timing of surgery (operation time) and the preoperative neurological status. They showed functional recovery to correlate inversely with the duration of spinal cord compression and the degree of severity. Our patients' symptoms evolved rapidly and by the time of surgical decompression, 24 hr after onset, he was completely paraplegic. There was no postoperative improvement. There is a evidence to suggest that functional recovery might have been better if the preoperative deficit was less severe.
Surgery performed more than 12 hr after symptom onset is unlikely to be successful (6). Immediate drainage is recommended by most surgeons. In our patients, no improvement in the neurologic status was seen after surgery. We thought that this unsuccessful result was due to the complete paraplegia preoperatively which was caused by delayed diagnosis.
The time interval between onset of symptoms and surgery is determined by how long it takes for a patient to recognize symptoms and enter into the medical system, and how long it takes for the physicians to recognize clinical signs and obtain radiological studies. Although physicians can do nothing about the patient's response time, they can accelerate the diagnostic evaluation, thereby minimizing the surgical delay.
The outcome is mainly depended on the time taken from symptom onset till operation, therefore early and accurate diagnosis such as careful history taking and immediate MRI evaluation is necessary. These symptoms initially appear as mild back pain and discomfort, which should not be ignored, instead close observation should be made to contribute to make early and accurate diagnosis.
Figures and Tables
Fig. 1
Saggital T2 weighted MR Image shows the heterogenous hyperintense lesion with focal areas of hypointensity posterior to the compressed spinal cord in T8-L2 level.
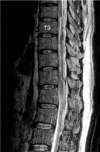
Fig. 2
Axial T2 weighted MR image shows the spinal cord compressed by the hyperintense lesion posterior to it in T10 level.

Fig. 3
Operative finding: Spinal cord is compressed by extensive blood clot and old hematoma. No other vascular abnormality.

Fig. 4
Saggital T2 weighted MR Image shows the heterogenous hyperintense lesion with focal areas of hypointensity posterior to the compressed spinal cord in T12-L2 level.
References
1. Awada A, Russell N, Fayez NA, Naufal R, Kohlani HA. Spontaneous cervical epidural hematoma: case report. Spinal Cord. 1998. 36:71–72.

2. Hayem G, Deutsch E, Roux S, Palazzo E, Grossin M, Meyer O. Spontaneous spinal epidural hematoma with spinal cord compression complicating plasma cell myeloma: a case report. Spine. 1998. 23:2432–2435.
3. Alexiadou-Rudolf C, Ernestus RI, Nanassis K, Lanfermann H, Klug N. Acute nontraumatic spinal epidural hematomas: An important differential diagnosis in spinal emergencies. Spine. 1998. 23:1810–1813.
4. Chang H, Bahk WJ, Park SA. Traumatic epidural hematoma of the cervical spine. A case report. J Korean Spine Surg. 1996. 3:262–266.
5. Lonjon MM, Paquis P, Chanalet S, Grellier P. Nontraumatic spinal epidural hematoma: report of four cases and review of the literature. Neurosurgery. 1997. 41:483–487.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download