Abstract
Blastic natural killer (NK) cell lymphoma is a rare neoplasm characterized by blastoid tumor cells expressing CD4 and CD56, with predominant skin involvement. Although this tumor has been regarded as a neoplasm related to NK cell, recent studies suggested that it is derived from plasmacytoid dendritic cells, but not from NK cell. Herein we report 4 cases of CD4+CD56+ lineage marker- blastic NK cell lymphomas with a review of literatures. The patients were 3 men and one woman. Three of them were young (17, 18, and 22 yr old). Three patients had skin lesions, at initial presentation in two patients and during the course of disease in other patient. Histologically, tumors consisted of monotonous medium to large blastoid cells showing no necrosis, angiocentric growth or epidermotrophism. All four tumors were CD4+ and CD56+. Three expressed CD68 antigen. Lineage specific markers for B- and T cell were negative. All tumors did not express myeloperoxidase. T-cell receptor gene rearrangement, EBV, CD13 and CD33 were negative. In one patient, tumor cells arranged in Homer-Wright type pseudorosette and expressed terminal deoxynucleotidyl transferase (TdT). Despite the standard lymphoma chemotherapy, the tumors, except one lost during follow-up, progressed and relapsed. The patients died 8-60 months after diagnosis.
The tumor cells of blastic NK cell lymphoma (Blastic NKL) express CD56 antigen with no obvious evidence of B- or T-cell differentiation and some cases expressing CD4 and TdT. Committed natural killer (NK) cell has been postulated as a cell of origin, because CD56 (NCAM) is useful marker in detecting NK-related cells (1). However, CD56 is not lineage-specific, and its aberrant expression also has been identified in a variety of unrelated hematopoietic malignancies including acute myeloid leukemia with myelomonocytic differentiation and other immature hematopoietic neoplasms of undefined origin. The results of recent studies challenged the terminology "blastic natural killer cell lymphoma" and support that blastic NKL is a tumor of plasmacytoid dendritic cells, not of NK progenitors (2). Herein we report 4 CD56+ CD4+ non-B, non-T, non-myeloid neoplasms with blastoid morphology.
A 45-yr-old man presented with multiple subcutaneous masses in the left upper and lower extremities. At that time, neither lymph nodal involvement nor leukemia was detected. The patient received 6 cycles of CHOP (cyclophosphamide, adriamycin, vincristine, prednisolone) and IMVP-16 (ifosfamide, methotrexate, etoposide, prednisolone), but the disease progressed. Multiple subcutaneous masses and lymph nodal invasion developed in 10 and 21 months after the first visit. He received adjuvant radiation therapy, but leukemic transformation (blast, 34% in peripheral blood smear) and brain involvement also occurred in 23 and 27 months after initial presentation, respectively. In spite of further chemotherapy with daunorubicin, Ara-C (cytosine arabinoside), vincristine and prednisolone as well as intrathecal chemotherapy, he died of sepsis 28 months after the diagnosis.
A 17-yr-old girl presented with multiple bluish and purpuric maculopatches on the upper and lower extremities and back, and a deep subcutaneous nodule for 2 months. Initial hematological findings were within normal limit. The patient was transferred to another hospital and further follow-up results were not available.
An 18-yr-old man was admitted with left inguinal lymphadenopathy, night sweat and weight loss. A computed tomographic scan of the abdomen revealed splenomegaly and the enlarged left para-aortic lymph nodes. Peripheral blood findings were as follows: hemoglobin (Hb) 15.5 g/dL; white blood cells (WBC) 5.4×103/µL; normal differential counts except increased monocytes (10.2%). The level of LDH (261 IU/L) and AST (144 U/L) increased. Bone marrow aspiration and biopsy showed no abnormal findings. He received 3 cycles of CHOP chemotherapy. Three months later, a cutaneous nodule in the left thigh developed. Subsequently he received additional 5 cycles of CHOP chemotherapy. Complete remission was achieved for 2 yr until he suffered from left mandibular pain. Extraction of a wisdom tooth followed by biopsy of the gum revealed atypical lymphoid infiltration. Two months later, he complained of headache and weight loss of 4 kg. On physical examination, protrusion of the right eyeball and enlargement of right neck lymph nodes were found. He was anemic (Hb 5.6 g/dL, Hct 15.7%, reticulocytes 0.08%) and had severe thrombocytopenia (93,000/µL) and leukopenia (480/µL). A computed tomographic scan of the head and neck showed a relatively well demarcated tumor in the right ethmoid sinus which extended into the right orbit, and the maxillary and frontal sinuses. Bone marrow biopsy was positive. Chemotherapy with IMVP-16 regimen was started. He died 60 months after the diagnosis.
A 22 yr-old male patient was admitted with the multiple lymphadenopathy of the inguinal, cervical, and preauricular lymph nodes, the largest of them measuring 4 cm in diameter. A computed tomographic scan of the abdomen revealed diffuse hepatosplenomegaly and the enlarged paraaortic, common iliac, and external iliac lymph nodes. Peripheral blood findings were as follows: Hb 15.3 g/dL; WBC 7.4×103/µL; normal differential except increased monocytes (8.2%). The level of LDH (676 IU/L) increased. The bone marrow was positive. After biopsy of the cervical lymph node, he received one cycle of CHOP chemotherapy without response. Then high dose CHOP chemotherapy was given with achievement of complete remission. Subsequently the patient underwent peripheral blood stem cell transplantation, however, the tumor recurred in the bone marrow four months later.
Tumors of all four cases displayed similar appearance and were composed of diffuse infiltration of monotonous medium-to-large blastoid cells. Nuclei of three cases showed fine chromatin and inconspicuous nucleoli. Tumor cells of Case 4 exhibited prominent nucleoli simulating immunoblast. In skin biopsy, dense infiltration of tumor cells without necrosis or angiocentric growth was noted in the upper and mid-dermis and occasionally in the dermis to the subcutaneous tissue (Fig. 1). Mitoses were rather variable. The tumor cells exhibited no epidermotrophism. Biopsy of lymph nodes showed effacement of nodal architecture by diffuse proliferation of monomorphic lymphoid cells. Peculiarly, in Case 3, tumor cells were arranged around a central fibrillary core, which was very similar to Homer-Wright type pseudorosettes seen in neuroepithelial tumors (Fig. 2A). Touch imprint with Giemsa stain revealed no cytoplasmic azurophilic granules.
Immunohistochemial findings and main clinical information of these cases are summarized in Table 1. CD2 (Novocastra, U.K.), CD3 (Novocastra, U.K.), CD4 (Novocastra, U.K.), CD5 (Novocastra, U.K.), CD7 (Novocastra, U.K.), CD8 (Novocastra, U.K.), CD20 (Novocastra, U.K.), CD30 (Dako, Denmark), CD34 (Dako, Denmark), CD43 (Serotechs, Bethesda, MD), CD56 (Monosan, The Netherland), CD68 (Dako), CD79a (Dako), terminal deoxynucleotidyl transferase (TdT, Dako), myeloperoxidase (Dako), TIA-1 (Immunotech, Westbrook, ME), lysozyme (Zymed, U.S.A.) were used in the present study. All four tumors were CD4+/CD56+ (Fig. 3). Only Case 3 tumor displayed positive reactivity for TdT (Fig. 2B). All four tumors were negative for myeloperoxidase. As for CD68, three cases were positive, while Case 3 was negative. Flow cytometric analysis was performed in Case 3 and 4. Myeloid markers (CD13, CD33) were negative. EBV in situ hybridization was performed using the fluorescein-conjugated EBER1 and 2 oligonucleotides (Dako, Denmark). Briefly, paraffin sections were pretreated with xylene, followed by the treatment with proteinase K and finally hybridized with EBV oligonucleotides. As negative controls we used EBV-negative lymphoid tissues and the hybridization mixture without the EBV oligonucleotides. The results were all negative.
For polymerase chain reaction amplification of the T-cell receptor (TCR) gamma gene, DNA was prepared by standard proteinase K digestion and phenol/chloroform extraction. PCR followed by SSCP analysis was performed as described previously (5). No clonal rearrangement of TCR gene was demonstrated in all four cases.
Blastic NK cell lymphoma has been described under the various names including lymphoblastic lymphoma of NK phenotype, blastoid NK cell lymphoma, and leukemic lymphoma of immature NK lineage, and recently defined as a distinct entity of unknown cellular origin in the new World Health Organization classification (1). The neoplastic cells are negative for surface CD3 and are positive for CD56. CD4 is usually expressed. CD68 is generally negative, or only weakly and focally expressed. Expression of CD2, cytoplasmic CD3ε, and cytotoxic molecules is variable, but usually negative. Some cases are TdT and/or CD34-positive. Diagnosis of blastic NKL can be made in the absence of commitment to the B-, T-cell or myeloid lineage. Thus the tumor should be negative for TCR gene rearrangement, surface CD3, myeloperoxidase, CD13 and CD33 (1). In the present study, all cases showed blastoid morphology and expressed CD56 and CD4 with absence of myeloperoxidase expression and TCR gene rearrangement, thus fulfilling the minimum criteria of blastic NKL in the current WHO classification. Similar to previous reports, two cases presented with skin lesions, but the others presented with lymphadenopathy. TdT expression was noted in only one case.
These blastic NKL raises two interesting questions. First, is the term, blastic NKL, appropriate to define these CD4+ CD56+ lineage-negative blastoid neoplasms? Second, what is the pathogenetic implication of TdT expression in blastic NKL?
In the normal developmental pathway of NK cells, pre-NK cells express CD161, immature NK cells express CD161 and CD56, and mature NK cells express CD161, CD56 and CD94. Whereas aggressive NK-cell leukaemia/lymphoma and nasal NK-cell lymphoma, express both CD56 and CD94 with strong NK activity, tumor cells of blastic NKL lack CD94 and CD161 and express CD56 only (6). That is, blastic NKL does not express any of these mature or immature NK-related markers except CD56. CD56 is not NK-lineage specific marker but often expressed on various types of neoplasia that are not of NK lineage (6). The only presence of CD56 without other evidence of NK lineage is not sufficient to support the notion that those tumors are of NK blast. Hence, some authors have objected to the term "Blastic NK lymphoma" and proposed other name about these CD56+ CD4+ non-B, non-T neoplasms.
The term "CD56 positive undifferentiated acute leukemia" was suggested by Almasri et al. who denied NK cell origin for this tumor because CD4 is not expressed on normal fetal or adult NK cells (7). On the other hand, Petrella et al. proposed "CD4+CD56+ hematodermic neoplasm", because these neoplasms mainly involve the skin (2). In addition, they asserted that CD4+CD56+ hematodermic neoplasm is a tumor of plasmacytoid dendritic cell (pDC) (2). Likewise Jacob et al. proposed "early plasmacytoid dendritic cell leukemia/lymphoma" for those CD4+CD56+ lineage-negative neoplasm (8).
Recently, Karube et al. divided non-B, non-T (lineage negative) neoplasms with lymphoblastic morphology into four types based on immunohistochemistry. They are 1) CD7+ stem cell lymphoma [CD4-, CD7+, CD33+/-, CD56-], 2) blastic NKL [CD4-, CD7+/-, CD33-, CD56+, CD123-], 3) myeloid/NK precursor cell leukemia [CD4-, CD7+, CD33+, CD56+], and 4) CD4+CD56+ hematodermic malignancy [CD4+, CD7+/-, CD33-, CD56+, CD123+]. Based on differences of clinical and phenotypical features, they contended that "CD4-56+ blastic NKL" is clearly distinct from CD4+ CD56+ hematodermic neoplasm (9).
pDC is an immature dendritic cell, and represents the key effector cells in the early antiviral innate immune response by producing large amounts of IFN-α/β upon viral infection. pDC induces CD13, CD33, and CD11c myeloid antigens when cultured in GM-CSF or IL-3. This lineage conversion supports the potential of pDCs to differentiate to myeloid lineage as observed in occasional development of acute myelomonocytic leukemia following cutaneous "blastic NKL" (10).
Another issue in blastic NKL is an expression of TdT in some but not all the cases. In the blastic NKLs reported previously, TdT was expressed in 19 of 31 CD4+CD56+ cases and 14 of 15 CD4-CD56+ cases (3, 9-24). In the study by Petrella et al. (2), all the 14 CD4+CD56+CD123+ hematodermic neoplasms were negative for TdT. Herling et al. showed 7 cases of CD4+CD56+CD123+ plasmacytoid dendritic cell tumors expressing TdT in variable patterns, and two of their patients had transformation to myelomonocytic leukemia (25). TdT is a marker for lymphoid blast and expressed in B- and T-lymphoblastic lymphoma, acute lymphoblastic leukemia, and lymphoblastic crisis of chronic myelogenous leukemia. Generally TdT is not expressed in cells of non-lymphoid malignancy, although approximately 20% of acute myeloid leukemia have TdT+ blasts, some of them express both myeloid markers and TdT. Even though there have been no data regarding TdT expression in normal precursors of NK cells and dendritic cells, in vitro studies showed that T, B, NK, and dendritic cells have a common progenitor in the extrathymic and thymic tissue (26, 27). In conjunction with development of myelomonocytic leukemia in some blastic NKL, expression of TdT in tumor cells suggests that blastic NKL is basically of multipotent cells which can differentiate into dendritic cell, myelomonocyte, and lymphoblast of either T cell or NK cell with the majority committed to plasmacytoid dendritic cell.
Histologically, our Case 3 exhibited rosette-like appearance. Homer-Wright rosettes consisting of neoplastic cells surrounding an eosinophilic fibrillary center without a lumen, is an important finding favoring a neuroepithelial tumor in the differential diagnosis of small round cell malignancy. In the malignant lymphoma, rosette-formation is a rare finding and has been reported in only a few cases.
As for treatment and prognosis, all three cases in the current study except one lost during follow-up, were treated with aggressive chemotherapy with or without radiation therapy. However, the outcomes were dismal. All three patients died from eight to sixty months after diagnosis. Clinical outcomes of our cases were similar to the known poor prognosis of CD56+ CD4+ non-B, non-T neoplasia reported previously (14, 28). Some other studies revealed that the patients treated with allogenic or autologous stem cell transplantation had a significantly more favorable prognosis than those treated with only chemotherapy and radiation therapy. This finding implies that intensive chemotherapy with stem cell transplantation might improve the prognosis of these types of blastoid lymphomas (8, 9)
In summary, we described clinicopathologic findings of 4 cases CD4+CD56+ non-B, non-T neoplasm (Blastic NKL by current WHO classification) which are distinct from other NK/T-cell lymphomas and show characteristic histological and immunophenotypic findings. Because the term "blastic NK cell lymphoma" seems to be a misnomer, we are looking forward to having an appropriate new terminology for this tumor in next WHO classification.
Figures and Tables
Fig. 1
Histologic finding of the skin biopsy (Case 1), dense infiltration of medium-sized blastoid tumor cells without necrosis or angiocentric growth noted in the upper and mid-dermis without epidermotrophism (H&E stain, A; ×100, B; ×250).
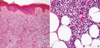
Fig. 2
In Case 3, tumor cells arranged in Homer-Wright type pseudorosette (A; H&E stain, ×400) with positivity for terminal deoxynucleotidyl transferase (B; ×400).
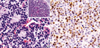
Fig. 3
Results of immunohistochemical stains. Most of tumor cells strongly express CD56 (A) and CD4 (B) (×400).

References
1. Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization Classification of Tumours, Pathology & Genetics Tumours of the Haematopoietic and Lymphoid Tissues. 2001. Lyon: International Agency for Research on Cancer (IARC) Press;214–215.
2. Petrella T, Comeau MR, Maynadie M, Couillault G, De Muret A, Maliszewski CR, Dalac S, Durlach A, Galibert L. 'Agranular CD4+ CD56+ hematodermic neoplasm' (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002. 26:852–862.

3. Ko YH, Kim SH, Ree HJ. Blastic NK-cell lymphoma expressing terminal deoxynucleotidyl transferase with Homer-Wright type pseudorosettes formation. Histopathology. 1998. 33:547–553.

4. Ko YH, Kim SH, Park K, Ree HJ. CD4+CD56+CD68+ hematopoietic tumor of probable plasmacytoid monocyte derivation with weak expression of cytoplasmic CD3. J Korean Med Sci. 2002. 17:833–839.
5. Signoretti S, Murphy M, Cangi MG, Puddu P, Kadin ME, Loda M. Detection of clonal T-cell receptor gamma gene rearrangements in paraffin-embedded tissue by polymerase chain reaction and nonradioactive single-strand conformational polymorphism analysis. Am J Pathol. 1999. 154:67–75.
6. Mori KL, Egashira M, Oshimi K. Differentiation stage of natural killer cell-lineage lymphoproliferative disorders based on phenotypic analysis. Br J Haematol. 2001. 115:225–228.

7. Almasri NM, Mitchell D, Braylan RC. Blastic natural killer cell. Am J Surg Pathol. 1999. 23:991–992.

8. Jacob MC, Chaperot L, Mossuz P, Feuillard J, Valensi F, Leroux D, Bene MC, Bensa JC, Briere F, Plumas J. CD4+ CD56+ lineage negative malignancies: a new entity developed from malignant early plasmacytoid dendritic cells. Haematologica. 2003. 88:941–955.
9. Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Suefuji H, Suzumiya J, Harada M, Kikuchi M. Non-B, non-T neoplasms with lymphoblast morphology: further clarification and classification. Am J Surg Pathol. 2003. 27:1366–1374.
10. Kazakov DV, Mentzel T, Burg G, Dummer R, Kempf W. Blastic natural killer-cell lymphoma of the skin associated with myelodysplastic syndrome or myelogenous leukaemia: a coincidence or more? Br J Dermatol. 2003. 149:869–876.

11. Shapiro M, Wasik MA, Junkins-Hopkins JM, Rook AH, Vittorio CC, Itakura H, Frankel MC, Georgala S, Schuster SJ. Complete remission in advanced blastic NK-cell lymphoma/leukemia in elderly patients using the hyper-CVAD regimen. Am J Hematol. 2003. 74:46–51.

12. Khoury JD, Medeiros LJ, Manning JT, Sulak LE, Bueso-Ramos C, Jones D. CD56 (+) TdT (+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002. 94:2401–2408.
13. Chang SE, Choi HJ, Huh J, Choi JH, Sung KJ, Moon KC, Koh JK. A case of primary cutaneous CD56+, TdT+, CD4+, blastic NK-cell lymphoma in a 19-year-old woman. Am J Dermatopathol. 2002. 24:72–75.

14. Feuillard J, Jacob MC, Valensi F, Maynadie M, Gressin R, Chaperot L, Arnoulet C, Brignole-Baudouin F, Drenou B, Duchayne E, Falkenrodt A, Garand R, Homolle E, Husson B, Kuhlein E, Le Calvez G, Sainty D, Sotto MF, Trimoreau F, Bene MC. Clinical and biologic features of CD4 (+) CD56 (+) malignancies. Blood. 2002. 99:1556–1563.
15. Aoyama Y, Yamane T, Hino M, Ohta K, Nakamae H, Yamamura R, Koh KR, Takubo T, Inoue T, Tatsumi Y, Tatsumi N. Blastic NK-cell lymphoma/leukemia with T-cell receptor gamma rearrangement. Ann Hematol. 2001. 80:752–754.
16. Bayerl MG, Rakozy CK, Mohamed AN, Vo TD, Long M, Eilender D, Palutke M. Blastic natural killer cell lymphoma/leukemia: a report of seven cases. Am J Clin Pathol. 2002. 17:41–50.
17. Rakozy CK, Mohamed AN, Vo TD, Khatib G, Long PM, Eilender D, Palutke M. CD56+/CD4+ lymphomas and leukemias are morphologically, immunophenotypically, cytogenetically, and clinically diverse. Am J Clin Pathol. 2001. 116:168–176.

18. Yamada O, Ichikawa M, Okamoto T, Park C, Motoji T, Mizoguchi H, Shibuya A. Killer T-cell induction in patients with blastic natural killer cell lymphoma/leukaemia: implications for successful treatment and possible therapeutic strategies. Br J Haematol. 2001. 113:153–160.

19. Liu Q, Ohshima K, Sumie A, Suzushima H, Iwasaki H, Kikuchi M. Nasal CD56 positive small round cell tumors. Differential diagnosis of hematological, neurogenic, and myogenic neoplasms. Virchows Arch. 2001. 438:271–279.
20. Kluin PM, Feller A, Gaulard P, Jaffe ES, Meijer CJ, Müller-Hermelink HK, Pileri S. Peripheral T/NK-cell lymphoma: a report of the IXth Workshop of the European Association for Haematopathology. Histopathology. 2001. 38:250–270.
21. Kojima H, Mukai HY, Shinagawa A, Yoshida C, Kamoshita M, Komeno T, Hasegawa Y, Yamashita Y, Mori N, Nagasawa T. Clinicopathological analyses of 5 Japanese patients with CD56+ primary cutaneous lymphomas. Int J Hematol. 2000. 72:477–483.
22. Isobe K, Tamaru JI, Nakamura S, Itami J, Aruga T, Uno T, Yasuda S, Mikata A, Ito H. Blastic natural killer cell lymphoma arising from the mediastinum with terminal deoxynucleotidyl transferase expression. Pathol Int. 2001. 51:55–59.

23. Koita H, Suzumiya J, Ohshima K, Takeshita M, Kimura N, Kikuchi M, Koono M. Lymphoblastic lymphoma expressing natural killer cell phenotype with involvement of the mediastinum and nasal cavity. Am J Surg Pathol. 1997. 21:242–248.

24. Adachi M, Maeda K, Takekawa M, Hinoda Y, Imai K, Sugiyama S, Yachi A. High expression of CD56 (N-CAM) in a patient with cutaneous CD4-positive lymphoma. Am J Hematol. 1994. 47:278–282.

25. Herling M, Teitell MA, Shen RR, Medeiros LJ, Jones D. TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood. 2003. 101:5007–5009.

26. Alonso-C LM, Munoz JJ, Zapata AG. Delineation of intrathymic T, NK, and dendritic cell (DC) progenitors in fetal and adult rats: demonstration of a bipotent T/DC intermediate precursor. J Immunol. 2001. 16:3635–3641.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download