Abstract
This study was conducted to examine lymphocyte subset counts and mood states in panic disorder patients. Twenty patients with panic disorder and 20 age- and gender-matched normal healthy subjects were recruited for the study. We used the Spielberger State (STAIS) & Trait (STAIT) Anxiety Inventory, Hamilton Depression Rating scale (HAMD) and Hamilton Anxiety Rating scale (HAMA) to measure mood states in all subjects. Lymphocyte subsets counts were made by flow cytometry. Panic patients showed significantly higher scores for anxiety and depression than normal subjects. Panic patients showed no differences in terms of the numbers of immune cells, as compared with normal healthy subjects, other than a lower proportion of T suppressor cells and a higher T helper cell/T suppressor cell ratio. HAMA and STAIS scores were common factors that could predict T cell numbers and proportions, T helper cell numbers, and natural killer cell proportions in panic disorder patients. We suggest that anxiety levels are related to the T-cell population in panic disorder patients and that quantitative immune differences may reflect altered immunity in this disorder.
An association between depression and altered immunity has been suggested by many research groups (1, 2), although immunological findings have not been consistently demonstrated. Immune changes associated with depression include decreased mitogen-induced lymphocyte proliferation and natural killer (NK) cell activity, and enumerative changes like increases in the total number of white blood cells and neutrophils, and a reduction in the number of lymphocytes (2, 3). Recently, depression is also thought to be associated with activation of some aspects of cellular immunity increasing secretion of proinflammatory cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor-alpha that can elicit depressive or anxiety symptoms, as contrasted with the initial opinion that depression can compromise immune system (4, 5).
Patients experiencing a major depressive episode along with a comorbid panic disorder have a greater number of T cells, and more mitogen-induced lymphocyte proliferation than depressed patients without panic disorder (6). Panic disorder comorbidity seems to contribute to immune variances in patients with major depressive disorder, and it seems reasonable to postulate that some immunological alterations may be attributed to panic disorder. However, relatively few studies have been carried out to observe immune changes in panic disorder, and these studies do not reveal any consistent immunological differences for panic disorder patients versus normal subjects. Marazziti et al. (7) showed that panic disorder patients do not differ from healthy controls in immune cell number, except for T helper (Th) cells, which were significantly lowered. However, many other studies reported no differences in the numbers and proportions of immunocytes in panic disorder without concurrent depression (8-10). In addition, some studies have indicated that B-cell numbers are altered in panic disorder patients, although not all in the same direction (10-12).
We postulated that panic disorder patients have altered immunity versus normal healthy subjects, and thus we examined differences in lymphocyte subset counts between panic disorder patients and control subjects. We also tried to identify the relationship between clinical variables including mood states, and lymphocyte subsets in panic disorder patients.
All subjects were medically healthy ones, and they included 20 panic disorder patients and 20 age- and gender-matched normal control subjects (10 males and 10 females in each group). The panic disorder patients who had other major psychiatric disorders including other anxiety disorders, major depressive disorder, and substance abuse were excluded from this study. The patients were new outpatients who had visited the Samsung Medical Center in Seoul, and all met the DSM-IV criteria for panic disorder using the Anxiety Disorder Interview Schedule for DSM-IV (13). Patients were not receiving any regular anti-panic medication at the time of study, although some of the patients took alprazolam intermittently at the time of study, but had never received any treatment for panic disorder previously. They had a relatively recent onset of illness (38.5±50.3 weeks), and 17 among 20 panic patients showed less than 1 yr of illness duration.
Physical examinations were performed with comprehensive history taking and some clinical laboratory tests (EKG, and liver function and thyroid function testing) so as to exclude panic symptoms secondary to other medical conditions. Healthy age- and gender-matched controls were recruited through an internet advertisement in Seoul. All subjects were required to be in good health, and this was defined as having no acute or chronic medical or psychiatric illness that could affect immunity (2, 14-17). Control subjects did not take any medication known to affect the immune system.
The Institutional Review Board of Samsung Medical Center approved the study protocol, and a written informed consent was obtained from all subjects after the procedures had been fully explained. Background data were collected, including demographic data and medical and psychiatric histories. Body Mass Index (BMI), which might affect immune function, was calculated as weight in kilograms divided by height in meters squared; BMIs ranged from 18 to 30 among all 40 subjects.
Subjects were instructed to fast for 12 hr prior to the study and to refrain from caffeinated beverages, alcohol, and smoking for 24 hr prior to the study. We began the study at 8 a.m., and upon arrival, a 20-gauge catheter was inserted into a forearm vein. After a physical examination and blood pressure monitoring, subjects were quietly rested for 30 min. At the end of this period, blood samples were collected. Four milliliters of blood was drawn initially and discarded and then at least 3 milliliters was drawn into an EDTA tube. The total white blood cell count and the differential count were obtained using a Coulter GEN-S hematology analyzer (Beckman Coulter, Inc., Fullerton, CA, U.S.A.). Whole blood samples were first stained with Simultest LeucoGATE, Control, and CD3/CD19, CD3/CD4, CD3/CD8, and CD3/CD16+CD56 reagents (BD Immunocytometry Systems, San Jose, CA, U.S.A.). Diluted FACS Lysing Solution (BD Immunocytometry Systems) was then used to lyse the red blood cells and stain. After appropriate washing and suspending, the cells were analyzed using a FACSort flow cytometer (BD Immunocytometry Systems). Gates were selected using forward and right angle light scatter and a minimum of 1,000 cells were included in each analysis. The instrument's photomultiplier tube voltages, fluorescence compensation levels, and sensitivity were always checked on the day of the analysis. After each of the subset proportions was enumerated from a two-color dot plot, absolute counts were calculated.
Psychological evaluations were done on all subjects on the same day of blood sampling. Anxiety symptoms were assessed using the Korean version of the Spielberger Trait (STAIT) & State (STAIS) Anxiety Inventory (18) and the Hamilton Anxiety Rating Scale (HAMA) (19), and depressive symptoms were assessed using the 17-item Hamilton Depression Rating Scale (HAMD) (20).
To compare immunological and psychological variables between panic patients and control subjects, independent t-tests were used. Pearson correlation analyses were done to assess whether clinical variables including anxiety or depressive levels are associated with immunologic alterations in patients with panic disorder. Stepwise multiple regression analyses were used to identify predictors of immune variables in panic disorder. All analyses were performed using SPSS 11.0 statistical software.
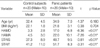
The characteristics of panic disorder patients and control subjects are shown in Table 1. No significant differences were evident with respect to age, sex, or BMI between the two groups. Panic disorder patients had significantly higher HAMA, HAMD, STAIS and STAIT scores than control subjects.
The patients with panic disorder had a lower T suppressor (Ts) cell proportion (t=2.63, p=0.012) and a higher Th/Ts ratio (t=-2.57, p=0.014), than controls. Total WBC number (t=-1.85, p=0.072) was slightly higher in panic disorder patients, but this did not reach statistical significance (Table 2).
Ten panic patients with a HAMD score of >17 had a lower BMI (p=0.031) than 10 panic disorder patients with a HAMD score of <17. More depressive panic patients had lower numbers (p=0.036) and proportions (p=0.013) of NK cell, but showed no differences with respect to other cumulative immune variables versus less depressive panic disorder patients.
Age, gender, and BMI showed no association with immune variables. For panic disorder patients, HAMA scores were positively correlated with T cell number (r=0.498, p=0.025), T cell proportion (r=0.495, p=0.026), and Th cell number (r=0.444, p=0.050), but HAMA scores correlated negatively with NK cell proportion (r=-0.529, p=0.016). STAIS scores for panic disorder patients showed a significant positive correlation with T cell proportion (r=0.533, p=0.023), and a negative correlation trend was noted between STAIS scores and NK cell proportion (r=-0.467, p=0.051). However, HAMD scores significantly negatively correlated with NK cell proportion (r=-0.449, p=0.047).
Stepwise multiple regression analyses were done to identify the clinical predictors of immune variable in panic disorder patients (Table 3). HAMA scores predicted T cell number (R2=0.296, p=0.020), Th cell number (R2=0.230, p=0.044), and NK cell proportion (R2=0.243, p=0.038), whereas STAIS scores predicted T cell proportion (R2=0.284, p=0.023). However, other clinical variables, including the level of depression, were not found to predict any immune parameter.
We found that panic disorder patients exhibited no differences with respect to the absolute number of immune cells, except for a reduced proportion of Ts cells and an increased Th/Ts ratio, as compared with normal healthy subjects. Marazziti et al. (7) reported significantly fewer Th cells and reduced Th/Ts ratios in panic disorder patients, and others have found increases (11, 12) or decreases (21) in B cell populations in panic disorder. However, our findings support a lack of a relation between this disorder and the absolute numbers of Th cells (9-12, 21), the B cells (7, 9), and NK cell (9, 10, 12, 21).
Suppressor T cells are known to suppress the action of other immune cells, most notably B-cells and T-cells, and thereby prevent the establishment of an immune response. Helper T cells act on other cells in the immune system to promote various aspects of immune response, including immunoglobulin isotype switching and affinity maturation of antibody response, macrophage activation, and the enhanced activity of NK cells and cytotoxic T cells (22). Thus, a reduction in the Ts cell proportion and an elevation of the Th/Ts ratio may reflect altered immunity, which suggests a possibility of T cell activation in panic disorder. Thus, examining the functional measures of T cell activation will be needed in a future study.
Some panic patients in this study had more depressive features (HAMD score >17), even though they did not meet the major depressive disorder DSM-IV criteria. Indeed, both panic disorder and major depressive disorders are highly comorbid and are probably affected by the same serotonin and norepinephrine neurotransmitter systems (23). However, patients with panic disorder are known to have immune findings that differ from those with major depression (6, 7). Moreover, the depressive symptoms of panic disorder patients were not found to affect any enumerative immune variables, except for NK cell proportion in this study. Thus, a depressed mood per se does not seem to play an important role in altered measures of immunity in panic disorder patients.
With regard to stress, a reduction in the number of lymphocytes and a suppression of lymphocyte proliferative response to mitogen were demonstrated by a psychiatric fellowship examination (24). Speech stress in healthy men was found to result in significant increases in Ts cells and NK cells and a reduction in the Th/Ts ratio versus baseline (25). Gerritsen and colleagues (26) also found that a laboratory induced fear situation (public speaking) produced changes in the experimental subjects' immune systems, and specifically reduced the numbers of Th cells and T lymphocytes. Heightened stress as well as heritable factors has been suggested to contribute to the onset of panic disorder (27). Although a panic attack itself can also be a severe stressor, previous findings described the above seem to be at odds with ours, even though we did not directly measure stress levels in this study. In the present study, we tried as much as possible to reduce the effects of acute stress on lymphocyte subsets during the experimental procedures. Thus, our findings concerning lymphocyte subsets are more likely to be related to panic disorder itself, and not stress per se.
Multiple regression analyses revealed that anxiety levels in panic disorder patients correlated positively with T cell number and proportion, and Th cell number, but anxiety levels were found to be negatively correlated with NK cell proportion in the present study. Weizman (28) reported that interleukin 3 (IL3) production is negatively correlated with the severity of anxiety, and that IL3 might be sensitive to the presence of anxiety. Hence, our findings also support that anxiety levels in panic disorder patients could affect immunity, although the mechanism and meaning of altered immunity in panic disorder remain unknown.
Several limitations of our study should be noted. First, the sample size in our study was relatively small, although there were very few immune studies with a large sample size in panic disorder. Second, there is a possibility that panic disorder patients with severe symptoms might have been excluded because the patients in this study had a relatively recent illness onset and did not take any regular medication at the time of study commencement. Third, our study was limited to enumerative immune measures, although recent immunological studies have included other functional immune measures, such as mitogen induced lymphocyte proliferation and NK cell cytotoxicity, as well as enumerative measures. In addition, the result of increased Th/Ts ratio in this study differed from that of our recent study (29), which showed no significant difference in Th/Ts ratio between panic disorder patients and normal control subjects. It is difficult to explain why this difference occurred, but one possible explanation would be that panic patients with different anxiety levels were involved in each study. Thus, in the future, a prospective study with a lager sample size using functional immune measures, as well as enumerative ones, will be needed to confirm the contradictory immune finding in panic disorder.
In conclusion, panic disorder patients showed a reduced Ts cell proportion and an increased Th/Ts ratio. Moreover, higher levels of anxiety were found to be related to an altered T cell population in panic disorder patients. Quantitative immune differences may reflect altered immunity in panic disorder.
Figures and Tables
ACKNOWLEDGEMENT
This work was supported by an SBRI grant (CA00143) from the Samsung Biomedical Research Institute. The authors are grateful to Kyungjeong Kim, B.Sc. and Changgon Lee, M.D. at the Psychiatric Department of Samsung Medical Center for determining lymphocyte subset patterns.
References
1. Stein M, Miller AH, Trestman RL. Depression, the immune system, and health and illness. Findings in search of meaning. Arch Gen Psychiatry. 1991. 48:171–177.
2. Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993. 113:472–486.

3. Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001. 15:199–226.

4. Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann Med. 2003. 35:2–11.

5. Leonard B. Stress, depression and the activation of the immune system. World J Biol Psychiatry. 2000. 1:17–25.

6. Andreoli A, Keller SE, Rabaeus M, Zaugg L, Garrone G, Taban C. Immunity, major depression, and panic disorder comorbidity. Biol Psychiatry. 1992. 31:896–908.

7. Marazziti D, Ambrogi F, Vanacore R, Mignani V, Savino M, Palego L, Cassano GB, Akiskal HS. Immune cell imbalance in major depressive and panic disorders. Neuropsychobiology. 1992. 26:23–26.

8. Surman OS, Williams J, Sheehan DV, Strom TB, Jones KJ, Coleman J. Immunological response to stress in agoraphobia and panic attacks. Biol Psychiatry. 1986. 21:768–774.

9. Manfro GG, Pollack MH, Otto MW, Worthington JJ, Rosenbaum JF, Scott EL, Kradin RL. Cell-surface expression of L-selectin (CD62L) by blood lymphocytes: correlates with affective parameters and severity of panic disorder. Depress Anxiety. 2000. 11:31–37.

10. Schleifer SJ, Keller SE, Scott BJ, Vecchione J. Lymphocyte function in panic disorder. Biol Psychiatry. 1990. 27:Suppl 9A. 66A.
11. Rapaport MH. Circulating lymphocyte phenotypic surface markers in anxiety disorder patients and normal volunteers. Biol Psychiatry. 1998. 43:458–463.

12. Perini GI, Zara M, Carraro C, Tosin C, Gava F, Santucci MG, Valverde S, De Franchis G. Psychoimmunoendocrine aspects of panic disorder. Hum Psychopharmacol. 1995. 10:461–465.

13. Brown T, DiNardo P, Barlow D. The Anxiety Disorder Interview Schedule for DSM-IV. Center for Stress and Anxiety Disorders. 1994. Albany, NY: State University of New York.
14. Mazur E, Wolski A. Cytokines: regulations of immune response during infection. Pol Merkur Lekarski. 2001. 11:375–377.
15. Singh B, Delovitch TL. Immune mechanisms that regulate susceptibility to autoimmune type I diabetes. Clin Rev Allergy Immunol. 2000. 19:247–264.

16. Rothermundt M, Arolt V, Bayer TA. Review of immunological and immunopathological findings in schizophrenia. Brain Behav Immun. 2001. 15:319–339.

17. Tsai SY, Chen KP, Yang YY, Chen CC, Lee JC, Singh VK, Leu SJ. Activation of indices of cell-mediated immunity in bipolar mania. Biol Psychiatry. 1999. 45:989–994.

18. Spielberger C, Gorsuch R, Lushene R. STAI manual for the statetrait anxiety inventory. 1970. Palo Alto: Consulting Psychologists Press.
21. Schleifer SJ, Keller SE, Bartlett JA. Panic disorder and immunity: few effects on circulating lymphocytes, mitogen response, and NK cell activity. Brain Behav Immun. 2002. 16:698–705.

22. Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998. 10:649–655.

23. Asnis GM, Wetzler S, Sanderson WC, Kahn RS, van Praag HM. Functional interrelationship of serotonin and norepinephrine: cortisol response to MCPP and DMI in patients with panic disorder, patients with depression, and normal control subjects. Psychiatry Res. 1992. 43:65–76.

24. Dorian BJ, Keysteon E, Garfinkel PE, Brown GH. Immune mechanism in acute psychological stress. Psychosom Med. 1981. 43:84.
25. Mills PJ, Haeri SL, Dimsdale JE. Temporal stability of acute stressor-induced changes in cellular immunity. Int J Psychophysiol. 1995. 19:287–290.

26. Gerritsen W, Heijnen CJ, Wiegant VM, Bermond B, Frijda NH. Experimental social fear: immunological, hormonal, and autonomic concomitants. Psychosom Med. 1996. 58:273–286.
27. Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000. 157:493–505.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download