Abstract
In Korea, common whelk (Buccinum undatum) is a popular edible shellfish. The aim of this study was to observe the sensitization rate to common whelk and to characterize its allergens. We carried out skin prick test (SPT) in 1,700 patients with various allergic diseases. Specific IgE were detected by ELISA in the patient sera and ELISA inhibition tests were conducted. IgE-binding components were identified by means of SDS-PAGE and IgE-immunoblotting. The effects of digestive enzymes were evaluated in both raw and thermally treated extracts. SPT to common whelk was positive (≥2+) in 83 (4.9%) patients studied. Twenty-four (38.7%) out of 62 SPT positive patients had high serum specific IgE to common whelk. ELISA inhibition test showed significant inhibitions by abalone as well as by common whelk. IgE-immunoblotting demonstrated three IgE-binding components (40, 71, 82 kDa), which were digested by simulated intestinal fluid and moderately digested by simulated gastric fluid, and the digestibility of allergens remained unchanged after thermal treatment. In conclusion, IgE-sensitization rate to common whelk was 4.9% in allergy patients. IgE-immunoblotting demonstrated three IgE-binding components, which were degraded by digestive enzymes. Further studies are needed to evaluate the clinical significance of the sensitized patients to common whelk.
Buccinum undatum, known as the common whelk or northern whelk, is one of the largest and most common sea snails in shallow coastal areas in North Atlantic (1). The common whelk is classified in the phylum Mollusca, class Mollusca, order Neogastropoda, family Buccinidae, and one of the popular edible shellfish in Korea. Hypersensitivity reactions, such as urticaria, angioedema, asthma, gastrointestinal distress and anaphylaxis have been described in patients consuming mollusks, in which common whelk belongs (2-7). Considering the huge amount of the common whelk consumption in Korea (8), it is likely that hypersensitivity reaction to common whelk is not rare. However, there was no report on hypersensitivity to common whelks and its allergenecity.
In this study, to evaluate the clinical significance of the common whelk as a food allergen, skin prick test (SPT) and specific IgE determination by ELISA were performed. To characterize IgE-binding components, 10% SDS-PAGE and IgE-immunoblot analysis were performed. In addition, their stability to digestive enzymes was evaluated.
Patients were selected from the Allergy Clinic of Ajou University Hospital in Suwon, Korea. Between January and December of 2001, 1,700 patients visited for treatment of various allergic diseases, including asthma, rhinitis, urticaria/angioedema, food allergies were skin tested. Four hundred twenty four patients, who had positive reactions to more than one food allergen, were tested whether they have serum specific IgE to various food allegens by method of ELISA. Sera from the patients showing strong positive responses (≥4+) to common whelk were used for the ELISA inhibitions test, IgE-immunoblotting and digestibility tests.
Common inhalant allergens for skin prick tests were purchased (Allergopharma Joachim Ganzer KG, Reinbek, Germany), and thirty food allergens commonly being consumed in Korea were prepared. Common whelk was kindly provided by a food processing company (Yusung Mulsan Co., Ltd., Seoul, Korea) as a fresh-frozen state. We prepared food allergen according to the method described previously (9). Briefly, foodstuffs (1:5 w/v) were homogenized in phosphate-buffered saline (PBS, pH 7.5). The homogenate was kept at 4℃ overnight followed by centrifugation at 3,500 rpm for 40 min. The supernatant was dialyzed (the cut-off molecular weight was 6,000 Da; Spectrum Medical Industries, Houston, TX, U.S.A.) against 4 L of PBS at 4℃ for 48 hr. The supernatant was passed through Nalgen syringe filter (Rochester, New York, NY, U.S.A.) and lyophilized until testing for ELISA, ELISA inhibition, immunoblotting analysis, and digestibility tests. For skin prick testing, the 1:5 w/v extract was mixed with an equal amount of glycerin (Sigma Co., St. Louis, MO).
Routine SPTs using 26-gage needles were performed on the backs of the patients simultaneously using 50 common inhalant allergens and 30 food allergens. The results were read 15 min later. The size of the wheal produced by each antigen and histamine was expressed as mean diameter of maximum diameter and vertical length at midportion of maximal length. Skin reactivity was expressed as the ratio of wheal size of allergen to histamine (A/H). If A/H was from 0.1 to 1 and erythema had a diameter <21 mm, it was read as 1+. If A/H was from 0.1 to 1, but erythema was >21 mm, it was read as 2+. If A/H ratio was 1 to 2, it was read as 3+. If A/H ratio was from 2 to 3, it was read as 4+. If A/H was greater than 3, it was read as 5+. A positive responder was defined as a responder who demonstrated a response greater than 2+ on SPT (10).
ELISA was performed to determine the presence of specific IgE to common whelk as previously described (11). Microtiter plates (Costar, New York, NY, U.S.A.) were coated with 100 µL/well of common whelk extract (diluted in carbonated buffer pH 9.6, 10 µg/well) and left at 4℃ for 24 hr. Each well was washed three times with 0.05% PBS-Tween (PBST), and the remaining binding sites were blocked by incubation with 350 µL/well 3% BSA-PBST for 1 hr at room temperature (RT). The wells were then incubated for 3 hr at RT with either 50 µL of the patient's or control sera (all sera was diluted 1 to 5). After washing three times with PBST, biotin-labeled goat anti-human IgE antibody (Vector Co, Burlingame, CA, U.S.A.) was added to the each well and incubated for 1 hr. After washing, the wells were incubated with 50 µL of 1:1,000 v/v streptavidin-peroxidase (Sigma Co., St. Louis, MO, U.S.A.) for 30 min before another washing step. Colorimetric reaction was developed with B (3,3',5,5'-tetramethylbenzidine) substrate solution for 10 min at RT. The reaction was stopped by the addition of 50 µL of 2 N sulfuric acid and the absorbance was read at 450 nm by an automated microplate reader (Benchmark, Bio-Rad, Hercules, CA, U.S.A.). All assays were performed in duplicate. Amounts of specific antibodies in the samples were calculated from control curves made by optical densities from serial dilutions of positive control samples which showed higher titer antibodies using a spline fit program (Microplate Manager, Bio-Rad) and expressed by arbitrary unit (A.U.). Specific IgE to common whelk was considered positive if the absorbance value was higher than the cutoff value, which was derived from mean +3×SD absorbance values from 15 control subjects.
Competitive ELISA inhibition was performed to determine the specificity of IgE-binding to the common whelk and to confirm cross-allergenecity with other food allergens. The sera from patients who had high specific IgE-binding were pooled and preincubated with four concentrations (5 to 100 µL/mL) of shrimp (Metapenaeus joyneri), avalone (Nordotis discus discus), house dust mite (Dermatophagoides pteronyssinus), chestnut and common whelk extracts, respectively, overnight at 4℃. Then, they were incubated with the common whelkcoated microtiter plate for 3 hr. The same steps of the experiments were followed as in ELISA. As a control, samples were preincubated with equal volumes of PBS (pH 7.5) instead of inhibitors, and the inhibition percent of the specific IgE-binding was expressed as (100-{[absorbance of samples preincubated with allergens/absorbance of samples preincubated with PBS]×100}).
Common whelk extract was electrophoresd in a 10% polyacrylamide separation gel with 4% stacking gel. Proteins from the gel were stained with colloidal blue (Novex, San Diego, CA, U.S.A.) or transferred onto a nitrocellulose membrane (Bio-Rad Laboratories), which was incubated with the pooled serum of patients with positive skin prick test diluted 1:5 v/v with PBS-Tween. Bound specific IgE was detected by biotin-labeled goat anti-human IgE antibody (Sigma) conjugated with streptavidine alkaline phosphatase (Sigma) followed by 0.66% 4-nitro-blue tetrazolium and 0.33% 5-bromo-4-chloro-3-indolyl-phosphate (Sigma), which was used as a substrate.
The preheated extracts were prepared by heating crude extracts at 100℃ for 5 min. The digestibility of the crude extract per se and preheated extract in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) was examined, as described (12, 13). Briefly, for the digestibility test in SGF, a protein sample (680 µg of nalve crude protein or preheated extract) was dissolved in 200 µL of prewarmed 100 mmol/L HCl, pH 1.2, with 30 mmol/L NaCl containing 0.32 w/v percentage of pepsin A (Sigma Chemical Co). Digestion was carried out at 37℃ with continuous shaking, and aliquots of this digest solution (20 µL) were withdrawn at 0, 0.5 and 60 min. These aliquots were quickly mixed with 26 µL of a sample buffer (containing 2% 2-mercaptoethanol and 4% SDS) together with 6.0 µL of Na2CO3 solution (200 mmol/L). The mixture was then boiled for 5 min and stored at -20℃ until further analyses. For the digestibility test in GIF, a sample (680 µg of nalve crude protein or preheated extract) was dissolved in 260 µL of prewarmed intestinal control solution (0.05 M KHSO4, pH 6.8) containing 1.0 w/v percentage of pancreatin (Pancreatin USP, Sigma Chemical Co.) and then incubated at 37℃ with continuous shaking. Aliquots of this digest solution (26 µL) were withdrawn at 1, 90 and 240 min and quickly boiled for 5 min after mixing with 26µL of a sample buffer (containing 2% 2-mercaptoethanol and 4% SDS). These pretreated digests were stored at -20℃ until further analyses.
Statistical analysis was performed with the SPSS 9.0 package for PCs (SPSS, Inc., Chicago, IL, U.S.A.). Correlations between specific IgE antibody level to common whelk with those of other foodstuffs were studied by Pearson's correlation. Correlations between skin test reactivities to foodstuffs were analyzed by Spearman rank order tests. Differences with p<.05 were considered to be significant.
The results of SPT throughout the year revealed that 83 (4.9%) of 1,700 patients showed more than 2+ of A/H to common whelk. The mean age of the 83 positive responders was 35.4±13.5 yr and 44 out of 83 patients were male. Among them, 2+, 3+, and 4+ responders, respectively, were 77 (4.5%), 4 (0.2%), and 2 patients (0.1%) as shown in Table 1.
ELISA on sera of 62 positive reactors as determined by SPTs, showed that the prevalence of specific IgE was 38.7% (24 out of 62 patients). When specifying to reactivity, the prevalence were 40.1% (23 out of 57 reactors) in 2+ reactors, 33.4% (1 out of 3 reactors) in 3+ reactors, and zero in two 4+ reactors (Fig. 1). Mean specific IgE values were 39.1±12.7, 336.2±330.7, 5.4±2.7 A.U., respectively, in 2+, 3+. 4+ reactors. Specific IgE levels were higher in positive SPT reactors to common whelk than those of controls (p<.05). However, no correlation was found between specific IgE levels and SPT reactivity.
Spearman rank order analysis of the correlation of SPT reactivities among food allergens showed no association between common whelk and spiny top shell (Batillus cornutus) (r=.082, p=.092), and positive associations between common whelk and abalone (Nordotis discus discus) (r=.105, p=.031). In addition, Pearson's correlation analysis of specific IgE levels revealed a significant association between common whelk and abalone (r=.58, p=.012) (Table 2).
ELISA inhibition tests using pooled sera were performed to evaluate cross-allergenecities between common whelk and other major food allergens. The test with common whelk extract showed significant dose-dependent inhibition. ELISA inhibition test with abalone showed 47.7% inhibition at 10 µg/mL, and 80.4% inhibition at 50 µg/mL, in a dose-dependent manner, whereas no inhibition was noted with house dust mite, shrimp, and chestnut allergens (Fig. 2).
To observe the protein components of common whelk, the extract was analyzed by a 10% SDS-PAGE and IgE-immunoblotting was applied with the patients' sera. As shown in Fig. 3, three IgE-binding components (40, 71, and 82 kDa) were detected within common whelk extracts, while no bindings were noted with control sera.
Most protein bands of common whelk extracts, preheated and non-treated, started to be digested by SGF in 5 min and were substantially digested within 1 and half hours in SDS-PAGE (Fig. 4). Immunoblotting using the pooled sera showed that most IgE-binding components were substantially digested by SGF within 1 hr in both preheated and non-heated conditions (Fig. 5). After the treatment of SIF, most protein and allergens within common whelk extracts were not observed in both preheated and non-treated conditions (Fig. 6).
There are over 80,000 living species of mollusks, which are second only to arthropods in the number and diversity of living species. Mollusks are classified into 3 classes; Class Mollusca (whelk and abalone), Class Bivalvia (mussel, scallop, and oyster), Class Cephlaopoda (squid and octopus). Common whelk is a boreal species, and common in coastal areas of the north Atlantic; in Europe it spans from Norway to Spain and from Labrador to New Jersey along the North American seaboard (1). Main markets of the European whelk fishery are Korea and Japan (14). In Korea, the amount of imported common whelk was 12,639 ton in 2001 (8).
Shellfish is well known cause of adverse food reactions in hypersensitive individuals. However, allergic reactions to mollusks have not been as well studied as those of crustaceans (15). Until now, several anaphylactic reactions have been described in patients consuming mollusks such as limpet (2), abalone (3, 4), snail (garden snail or vineyard snail) (5), mussel (6), and squid (7). Most of the reports were done in the geographic regions where mollusks have gained widespread culinary acceptance. The Koreans eat various shellfish. The annual shellfish supply was 50,100 ton (10.6 kg per person) in 2001 (8). Although there has been no report of allergic reactions to mollusks in this country, it is very likely that these adverse reactions are not rare.
In this study, the prevalence of positive responses on SPT results was 4.9% in patients with various allergic diseases. High serum specific IgE levels were detected in patients with positive SPT. These findings suggest that we should consider common whelk as a causative food allergen in this country, although there was no reported case of common whelk allergy.
There have been several reports to suggest molecular mass of mollusks allergens ranging from 24 to >95 kDa. In this study, the common whelk allergens are 40, 71, and 82 kDa proteins. Lopata et al. reported that abalone (Haliotis midae) allergens are 38 and 49 kDa proteins (4), while Choi et al. reported that abalone allergens derived Korean market are 40, 60, 92 kDa proteins (16). According to Miyazawa et al. squid (Todarodes pacificus) allergen is a 38 kDa protein and the amino acid sequence analysis of the allergen showed a marked homology with tropomyosin from blood fluke planorbid (Biomphalaria glabrata) (17). Among the various allergen molecules, tropomyosin has been focused. Tropomyosins are a group of highly conserved proteins present in muscle and non-muscle cells, and play a central role in muscle contraction (18). Thus similar, if not identical, allergenic epitopes may be present on tropomyosin of crustaceans and mollusks (19, 20).
In this study, cross-allergenecity between common whelk and abalone was suggested on ELISA inhibition test, which can be explained by their close taxonomic relationship. No cross-allergenecity was found with shrimp and with HDM, which was different result to the previous studies, which showed cross-allergenecity between mollusks and crustaceans (15, 19, 21). It may be caused by that the allergen extracts used in this study was different from that of other studies.
We evaluated the effect of digestive enzymes on IgE-binding components. In this study, common whelk extracts were digested rapidly in SIF and moderately in SGF. Although heating had minimal effects on IgE-binding components, they were degraded by both SGF and SIF. Generally, it was suggested that food allergens with high allergenecity are more resistant to SGF. It would be difficult to rank the allergenic potential of proteins on the basis of their SGF or SIF digestibility (12). There is a study suggesting that the lack of detection of SGF-digested products from an allergen by protein staining or IgE-immunoblotting after SDS-PAGE fractionation does not necessarily mean its inactivation in SGF (22). Further studies in a larger scale of patients will be needed to make any conclusion with digestibility test with SGF and SIF on common whelks allergens.
In conclusion, sensitization rate to common whelk in the patients with allergic diseases was 4.9%. Three IgE-binding components (40, 71, 82 kDa) were identified within common whelk extracts, which could be degradated by digestive enzymes. Further studies are needed to elucidate clinical significance of the sensitized patients to common whelk.
Figures and Tables
Fig. 1
Serum specific IgE-bindings to common whelk by ELISA according to A/H ratio on skin prick test. Transverse dotted line indicate the cut-off value of positive specific IgE level which were derived from mean +3×S.D. of absorbance values of controls.

Fig. 2
Percent inhibitions of specific IgE-bindings to common whelk with serial additions of common whelk (▪), shrimp (▴), abalone (•), D. pteronyssinus (▵), and chestnut (○) extracts using pooled sera from three patients having high specific IgE to common whelk.

Fig. 3
SDS-PAGE and IgE-immunoblotting of the common whelk extract in sera of the sensitized patients. Lane M: molecular weight markers; Lane 1: SDS-PAGE of 10 µg/mL of the raw common whelk extracts; Lane 2-5: IgE-immunobloting in sera from the patients group; Lane 6 and 7: IgE-immunobloting in sera from normal control group; Lane 8: buffer control.

Fig. 4
SDS-PAGE of SGF treated raw and preheated common whelk extracts. (A) SDS-PAGE analysis of the SGF digestion of raw extract. Lane M: molecular weight markers; Lane 1-8: SGF digestion pattern at t=0, 0.5, 1, 2, 5, 10, 30, and 90 min. (B) SDS-PAGE analysis of the SGF digestion of preheated extract. Lane M: molecular weight markers; Lane 1-8: SGF digestion pattern at t=0, 0.5, 1, 2, 5, 10, 30, and 90 min.
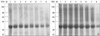
Fig. 5
SDS-PAGE and IgE-immunoblotting of SGF treated raw and preheated common whelk extracts in sera of the sensitized patients. (A) SDS-PAGE analysis of the SGF treated extracts. Lane M: molecular weight markers; Lane 1-3: SGF digestion pattern of raw extract at t=0, 0.5 and 60 min; Lane 4-6: SGF digestion pattern of preheated extract at t=0, 0.5 and 60 min. (B) IgE-immunoblotting of the SGF treated extracts. Lane M: molecular weight markers; Lane 1-3: IgE-immunoblotting of raw extract at t=0, 0.5 and 60 min; Lane 4-6: IgE-immunoblotting of preheated extract at t=0, 0.5 and 60 min.

Fig. 6
SDS-PAGE and IgE-immunoblotting of SIF treated raw and preheated common whelk extracts in sera of the sensitized patients. (A) SDS-PAGE analysis of the SIF treated extracts. Lane M: molecular weight markers; Lane 1: SIF; Lane 2: SIF untreated raw extract; Lane 3-5: SIF digestion pattern of raw extract at t=1, 90 and 240 min; Lane 6: SIF untreated preheated extract; Lane 7-9: SIF digestion pattern of preheated extract at t=1, 90 and 240 min. (B) IgE-immunoblotting of the SIF treated extracts. Lane M: molecular weight markers; Lane 1: SIF; Lane 2: SIF untreated raw extract; Lane 3-5: SIF digestion pattern of raw extract at t=1, 90 and 240 min; Lane 6: SIF untreated preheated extract; Lane 7-9: SIF digestion pattern of preheated extract at t=1, 90 and 240 min.

ACKNOWLEDGMENT
We thank Yusung Mulsan Co. (Seoul, Korea) for providing the fresh-frozen common whelk used in this study.
References
1. Valentinsson D. Reproductive cycle and maternal effects on offspring size and number in the neogastropod Buccinum undatum (L). Marine Biol. 2002. 140:1139–1147.
2. Carrillo T, Rodriguez de Castro F, Blanco C, Castillo R, Quiralte J, Cuevas M. Anaphylaxis due to limpet ingestion. Ann Allergy. 1994. 73:504–508.
3. Morikawa A, Kato M, Tokuyama K, Kuroume T, Minoshima M, Iwata S. Anaphylaxis to grand keyhole limpet (abalone-like shell-fish) and abalone. Ann Allergy. 1990. 65:415–417.
4. Lopata AL, Zinn C, Potter PC. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J Allergy Clin Immunol. 1997. 100:642–648.

5. Amoroso S, Cocchiara R, Locorotondo G, Parlato A, Lampiasi N, Albeggiani G, Falagiani P, Geraci D. Antigens of Euparipha pisana (snail). I. Identification of allergens by means of in vivo and in vitro analysis. Int Arch Allergy Appl Immunol. 1988. 85:69–75.
6. Nettis E, Pannofino A, Dambra P, Loria MP, Di Maggio G, Damiani E, Ferrannini A, Tursi A. IgE-mediated urticaria/angioedema after ingestion of mussels. Acta Derm Venereol. 2001. 81:62.
7. Carrillo T, Castillo R, Caminero J, Cuevas M, Rodriguez JC, Acosta O, Rodriguez de Castro F. Squid hypersensitivity: a clinical and immunologic study. Ann Allergy. 1992. 68:483–487.
8. Korea Rural and Economic Institute. Annual food demand and supply in 2001 [in Korean]. 2001. 129–147.
9. Lee SK, Jee YK, Kim YK, Suh JH, Lee MH, Park HS. Identification of IgE binding components of Tetranychus urticae: species-specific and cross-reacting allergens with house dust mite [in Korean]. Korean J Asthma Allergy Clin Immunol. 2000. 20:879–886.
10. Park HS. Allergy skin tests in clinical practice [in Korean]. J Asthma Allergy Clin Immunol. 2001. 21:115–119.
11. Kim YK, Park HS, Kim HA, Lee MH, Choi JH, Kim SS, Lee SK, Nahm DH, Cho SH, Min KU, Kim YY. Two-spotted spider mite allergy: immunoglobulin E sensitization and characterization of allergenic components. Ann Allergy Asthma Immunol. 2002. 89:517–522.

12. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996. 14:1269–1273.

13. Yagami T, Haishima Y, Nakamura A, Osuna H, Ikezawa Z. Digestibility of allergens extracted from natural rubber latex and vegetable foods. J Allergy Clin Immunol. 2000. 106:752–762.

14. Tjärnö Marine Biological Laboratory, Stromstad, Sweden. Aquascope: Common whelk. accessed June 5, 2003. http://www.vattenkikaren.gu.se/fakta/arter/mollusca/prosobra/buccunda/buccu12e.html.
15. Daul CB, Morgan JE, Lehrer SB. Hypersensitivity reactions to crustacea and mollusks. Clin Rev Allergy. 1993. 11:201–222.
16. Choi JH, Yoon SH, Suh YJ, Suh CH, Nahm DH, Kim YK, Min KU, Park HS. Measurement of specific IgE to abalone (Haliotis discus hannai) and identification of IgE binding components [in Korean]. J Asthma Allergy Clin Immunol. 2003. 23:349–357.
17. Miyazawa H, Fukamachi H, Inagaki Y, Reese G, Daul CB, Lehrer SB, Inouye S, Sakaguchi M. Identification of the first major allergen of a squid (Todarodes pacificus). J Allergy Clin Immunol. 1996. 98:948–953.

18. Smillie LB. Preparation and identification of alpha- and beta-tropomyosins. Methods Enzymol. 1982. 85:234–241.
19. Leung PS, Chow WK, Duffey S, Kwan HS, Gershwin ME, Chu KH. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: evidence for tropomyosin as the common allergen. J Allergy Clin Immunol. 1996. 98:954–961.

20. Asturias JA, Eraso E, Arilla MC, Gomez-Bayon N, Inacio F, Martinez A. Cloning, isolation, and IgE-binding properties of Helix aspersa (brown garden snail) tropomyosin. Int Arch Allergy Immunol. 2002. 128:90–96.
21. Sidenius KE, Hallas TE, Poulsen LK, Mosbech H. Allergen cross-reactivity between house-dust mites and other invertebrates. Allergy. 2001. 56:723–733.

22. Diaz-Perales A, Blanco C, Sanchez-Monge R, Varela J, Carrillo T, Salcedo G. Analysis of avocado allergen (Prs a 1) IgE-binding peptides generated by simulated gastric fluid digestion. J Allergy Clin Immunol. 2003. 112:1002–1007.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download