Abstract
Gastrointestinal stromal tumors (GISTs) of the gallbladder are representative of an extremely rare group of tumors. We have encountered a patient with a malignant GIST of the gallbladder and presented it with a review of some articles. A 72-yr-old woman initially presented with right upper quadrant abdominal pain, fever and chills. Emergency cholecystectomy was performed under the impression of gallbladder empyema. Liver metastasis was found at 7 months postoperatively and the patient expired 9 months after the surgery. At the time of cholecystectomy, the gallbladder showed a necrotic serosal surface with an irregular thickened wall. A mass, 6 cm in length and 3 cm in width, encircled the whole wall of the neck and upper body of the gallbladder. Microscopic findings revealed frequent mitotic figures (more than 20/50 HPF) and tumor necrosis. Hyperchromatic, pleomorphic and spindle shaped neoplastic cells that were arranged in a pattern of short fascicles infiltrated the entire layer of the gallbladder. The tumor cells were immunoreactive for CD117 antigen (c-kit protein) and vimentin. They were negative for desmin, smooth muscle actin and S-100 protein. Mutations of the c-kit proto-oncogene were not found in this case. These findings were sufficient to provide enough clinical, histopathological and immunohistochemicalevidence in diagnosing our case as a malignant GIST.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract and may arise anywhere in the tubular GI tract from the esophagus to the rectum. In terms of a more detailed distribution, 50-60% of the lesions arise in the stomach, 20-30% in the small bowel, 10% in the large bowel and 5% in the esophagus (1). In addition, it has been reported in recent years that identical lesions also occur in the mesentery, omentum and retroperitoneum (2). The demonstration of KIT expression in these lesions has helped to validate their existence, particularly in exceptional sites such as the gallbladder (3, 4) or urinary bladder (5). Recent studies found that GISTs are tumors originating from the interstitial cells of Cajal (ICCs) or primitive stem cells related to ICCs (6, 7). The morphologic appearance of stromal tumors in the gallbladder is identical to that of stromal tumors in the gastrointestinal tract, and ICCs are normally distributed in the gallbladder wall (8).
The prognosis of GISTs is difficult to predict due to various clinical patterns and morphologic characteristics and is poor when malignant changes are present or when metastasis occurs to the liver or peritoneal cavity in the early stage (9). Although GISTs of the gallbladder are extremely rare, we encountered a case of malignant GIST of the gallbladder that showed no mutation of c-kit proto-oncogene but had CD117 antigen expression. Thus, we report this case with the literature review.
A 72-yr-old woman visited St. Paul's Hospital of the Catholic University of Korea due to symptoms of fever, chills, and intermittent abdominal pain in the right upper quadrant that was present for 10 days and which worsened severe on the day of the visit. Ultrasound examination revealed a significantly dilated gallbladder and wall thickening. Several high echogenic foci with low echoic debris were present in the dependent portion of the gallbladder (Fig. 1). Accordingly, emergency cholecystectomy was performed under the diagnosis of gallbladder empyema.
Grossly, the gallbladder was dilated and measured 11 cm in length and 5 cm in width. A necrotic serosal surface that was purplish brown in color was observed. Upon opening, the gallbladder wall was thickened and yellowish necrotic tissue was present. A 6 cm long, 3 cm wide mass covered the entire wall of the gallbladder neck and body. Hemorrhage and necrosis were present in the remainder of the mucosal layer.
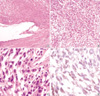
Microscopically, tumor cells were present in the entire layer of the gallbladder, and the mucosal layer was flattened due to tumor cells infiltration (Fig. 2A). Most of the tumor cells that were of spindle shape nature with some showing a round to oval appearance arranged in a short fascicle pattern (Fig. 2B). Hyperchromatic nuclei and severe pleomorphism were present in these cells. There was increased mitotic activity with mitotic counts being higher than 20/50 HPF (Fig. 2C), and partially tumor necrosis was also observed. Immunohistochemically, these tumor cells were stained diffusely with moderate intensity for CD117 (dilution 1:50, sc-168, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) in the cytoplasm (Fig. 2D) and they were positive for vimentin (dilution 1:25, V9, Signet Laboratories Inc., Dedham, MA, U.S.A.). However, they were negative for CD34 (dilution 1:50, N1632, DAKO, Carpinteria, CA, U.S.A.), desmin (dilution 1:40, ZC-18, ZYMED, San Francisco, CA, U.S.A.), smooth muscle actin (dilution 1:100, 1A4, NeoMarkers, Fremont, CA, U.S.A.) and S-100 protein (dilution 1:50, 4C4.9, NeoMarkers).
In order to determine the presence of c-kit gene mutation, sample sections were deparaffinized and DNA was extracted using the QIAamp DNA Mini Kit (QIAGEN Inc., Valencia, CA, U.S.A.) according to the proteinase K digestion methods. Polymerase chain reaction (PCR) was performed using the primers for 4 regions of the c-kit gene, i.e., exons 9, 11, 13, and 17. After the QIAquick PCR purification method (QIAGEN Inc.), the DNA sequence was analyzed using ABI 3700 sequencer (Applied Biosystems, Foster City, CA, U.S.A.) with the PCR products. DNA sequencing performed to determine the presence of the c-kit gene mutation revealed no mutation in exons 9, 11, 13, and 17.
Liver metastasis was found in abdominal CT (Fig. 3) at 7 months postoperatively and the patient expired 9 months after the surgical treatment.
Malignant stromal tumors of the gallbladder are extremely rare. Although some cases of leiomyosarcoma, botryoid rhabdomyosarcoma, angiosarcoma, and Kaposi sarcoma have been occasionally described (10), only two benign and malignant stromal tumors of the gallbladder with ICCs phenotype have been reported (3, 4).
The gallbladder tumor in our case was a high risk GIST as classified according to the risk of aggressive behavior of GIST proposed by Fletcher et al. in the NIH consensus symposium of GIST in 2001 (11). It has been expressed that the classification of GISTs into benign and malignant categories should be replaced by one that categorizes it into low-risk and high-risk tumors depending on their estimated potential for recurrence and metastasis (12). This proposal was made because lesions that are very small (less than 2 cm) and possess very low mitotic rates (less than 5/50 HPF) occasionally metastasize. With prolonged follow-up, it appears that almost all GISTs presenting with clinical symptoms or signs which lead to treatment have the potential to behave in a malignant fashion (11). The tumor in this report was 6 cm in length and 3 cm in width and had mitotic counts of more than 20 mitoses/50 HPF. It consisted of spindle cells most of which had hyperchromatic nuclei, along with significant pleomorphism and tumor necrosis. Actin, desmin and S-100 protein expression were absent but CD117 and vimentin expression were present. CD117 antibody is used to detect the KIT receptor protein and stain ICCs.
GISTs have been proposed to originate from ICCs because of the expression of KIT protein (7). KIT protein is also known to be expressed in several cell types, such as hematopoietic stem cells, mast cells, melanocytic cells, and germ cells and is considered to play an important role in the development and maintenance of these cellular elements (6). ICCs can easily be identified by immunohistochemical staining since they only express CD117, CD34 and vimentin in the gut (7). ICCs are pacemaker cells forming the nerve network that controls peristaltic movements of the GI tract and are located in the myenteric plexus and dispersed between the smooth muscle cells. It has been reported that the expression of activated KIT protein plays a crucial role in the differentiation and proliferation of ICCs (13).
KIT protein is a 145 kDa transmembrane receptor for a growth factor termed stem cell factor and it has an internal tyrosine kinase component (3, 14). The extracellular portion of KIT receptor binds to a ligand known as stem cell factor, and the intracellular portion contains the actual kinase enzymatic domain (15). KIT activation normally occurs when two adjacent receptors are brought together through binding to ligand dimers (15). However, the oncogenic activation of KIT protein, which is evoked by the oncogenic mutation of the c-kit gene itself, does not depend on binding of the KIT ligand, but instead plays a pivotal event in GIST tumorigenesis by inducing the phosphorylation of other proteins during the cell-signaling cascades (16). Therefore KIT expression might be one of the earliest transforming events in GISTs (17).
Many parameters such as tumor size, mitotic rate, mucosal invasion, tumor necrosis, high cellularity, anatomic site, and age have been proposed in predicting the prognosis of GISTs. However, the morphologic features that have gained greatest acceptance as being positive predictors of outcome are tumor size and mitotic rate (11, 12). According to recent reports, c-kit mutation results in an increase in both tumor size and mitotic rate leading to a clinically unfavorable prognosis (1). Lasota et al. (2) and Taniguchi et al. (18) suggested that mutation-positive GISTs showed a higher recurrence rate and death rate than mutation-negative GISTs and that the c-kit mutation was a strong candidate marker for predicting prognosis. On the contrary, Sakurai et al. (19) stated that the relationship between c-kit mutation and a poor prognosis of GISTs could not be confirmed. Similarly, although Wardelmann et al. (20) reported that there was correlation between the c-kit mutation and increased mitoses, but there was no statistical significance of the mutation as a prognostic factor. Even though identification of the specific c-kit mutation might be important in predicting clinical outcome and response to the KIT-specific therapy (14), data on the prognostic value of c-kit mutations are currently somewhat contradictory (1, 2, 18, 19) and their therapeutic significance still remains under investigation.
C-kit activating mutation was originally reported to be located in the juxtamembrane (exon 11) domain of the gene (17). However, subsequent studies revealed that mutation was in the extracellular (exon 9) and intracellular tyrosine kinase (exon 13 and 17) domains (16). In the case of GISTs, mutation usually occurs at exon 11, but when it does not occur at exon 11, rare mutations occur at exon 9 and exon 13 (14, 16). The frequency of c-kit mutation varies widely from 15% to 71% (18), at an average between 50-60% (2, 18, 19). However, we found no mutation in our case.
Diagnosis of the GIST of the gallbladder is not surprising because the gallbladder is a part of the tubular GI tract, having a common embryologic derivation. It has recently been shown that neoplasms phenotypically identical to gastrointestinal tumors occur as primary tumors in extragastrointestinal locations, principally in the omentum and mesentery (21). Our case of malignant GIST of the gallbladder, which express KIT protein while lacks the c-kit mutation, is histopathologically high risk as well as clinically malignant in nature due to the presence of liver metastases.
Figures and Tables
Fig. 1
Preoperative ultrasound reveals a significantly dilated gallbladder with wall thickening. Several high echogenic foci with low echoic debris (arrow) are present in the dependent portion of the gallbladder.

Fig. 2
(A) The wall of gallbladder is replaced by hypercellular spindle cells and the mucosal layer epithelium has been flattened by tumor infiltration (H&E, ×40). (B) Spindle cells are interspersed with fascicle patterns (H&E, ×100). (C) Higher magnification of spindle cells shows hyperchromasia and prominent nucleoli of nuclei, and mitoses are frequently noted (H&E, ×400). (D) Tumor cells are stained diffusely and show moderate intensity for CD117 in the cytoplasm (Immunostain ×400).
References
1. Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ. Prognosis of gastrointestinal smooth muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999. 23:82–87.
2. Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999. 154:53–60.

3. Mendoza-Marin M, Hoang MP, Albores-Saavedra J. Malignant stromal tumor of the gallbladder with interstitial cells of Cajal phenotype. Arch Pathol Lab Med. 2002. 126:481–483.

4. Ortiz-Hidalgo C, de Leon Bojorge B, Albores-Saavedra J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: a previously unrecognized neoplasm. Am J Surg Pathol. 2000. 24:1420–1423.
5. Lasota J, Carlson JA, Miettinen M. Spindle cell tumor of urinary bladder serosa with phenotypic and genotypic features of gastrointestinal stromal tumor. Arch Pathol Lab Med. 2000. 124:894–897.

6. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998. 152:1259–1269.
7. Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999. 23:377–389.

8. Albores-Saavedra J, Henson DE, Sobin LH. Histological Typing of Tumors of the Gallbladder and Extrahepatic Bile Ducts. World Health Organization. 1991. Berlin: Springer-Velag.
9. Hjermstad BM, Sobin LH, Helwig EB. Stromal tumors of the gastrointestinal tract: myogenic or neurogenic? Am J Surg Pathol. 1987. 11:383–386.

10. Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of the Gallbladder, Extrahepatic Bile Duct, and Ampulla of Vater. 2000. Washington, DC: Armed Forces Institute of Pathology;Atlas of Tumor Pathology, 3rd series, fascicle 27.
11. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002. 33:459–465.

12. Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: Recent advances in understanding of their biology. Hum Pathol. 1999. 30:1213–1220.

13. Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002. 33:484–495.

14. Longley BJ, Reguera MJ, Ma Y. Classes of c-Kit activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001. 25:571–576.

15. Blume-Jensen P, Claesson-Welsh L, Siegbahn A, Zsebo KM, Westermark B, Heldin CH. Activation of the human c-Kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991. 10:4121–4128.

16. Lux ML, Rubin BP, Biase TL, Chen CJ, Maclure T, Demetri G, Xiao S, Singer S, Fletcher CD, Fletcher JA. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000. 156:791–795.

17. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998. 279:577–580.

18. Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999. 59:4297–4300.
19. Sakurai S, Fukasawa T, Chong JM, Tanaka A, Fukayama M. C-kit gene abnormalities in gastrointestinal stromal tumors (tumors of interstitial cells of Cajal). Jpn J Cancer Res. 1999. 90:1321–1328.
20. Wardelmann E, Neidt I, Bierhoff E, Speidel N, Manegold C, Fischer HP, Pfeifer U, Pietsch T. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002. 15:125–136.

21. Lee CY, Kim JC, Kim WW, Chin HM, Kim W, Park CH, Jeon HM, Park SM, Lim KW, Park WB, Kim SN, Lee GY, Park GS, Song DY, Joo JH. Clinical effectiveness of diagnosis using immunohistochemistry and new grade in gastrointestinal stromal tumors (GISTs). J Korean Surg Soc. 2003. 64:471–479.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download