Abstract
We present a case with decreased metabolic activity of CYP2D6, a cytochrome P450 enzyme catalyzing the metabolism of nortriptyline (NT). Conventional dosage regimen led to toxic plasma concentration of NT and adverse effects such as dry mouth, constipation, and dizziness in this case with genotype CYP2D6*5/*10B. This case suggests the clinical usefulness of pharmacogenetic testing in individualized dosage adjustments of NT.
Nortriptyline (NT) has been used to treat various forms of depression, often in conjunction with psychotherapy. Common adverse effects associated with tricyclic antidepressant (TCA) therapy include dry mouth, blurred vision, tachycardia, constipation, urinary retention, sedation, and orthostatic hypotension (1, 2). These usually occur in association with toxic concentration of TCA.
NT is extensively metabolized in the liver. The main metabolic pathway of NT is benzylic hydroxylation in the C-10 position, mediated by CYP2D6 (1-3). Genetic polymorphism of CYP2D6 is responsible for the great interindividual variability in the pharmacokinetics of NT. CYP2D6 poor metabolizers may experience severe adverse effects (4-7), because of high NT concentrations. Alternatively, NT may be ineffective in ultrarapid metabolizers of CYP2D6 (4).
In this report, we present a case on NT therapy with CYP2D6*5/*10B, which results in decreased activity of CYP2D6. High plasma NT concentrations associated with adverse effects after standard doses of NT was found in this case.
A 54-yr-old man was hospitalized following a suicide attempt. For the last 2 months the patient had suffered from depression, anxiety, insomnia, and delusion. At the time of admission, physical examination of this man showed normal blood pressure, heart rate, and body temperature. His body weight was 66.8 kg and he was 165.5 cm in height. EKG finding was unremarkable. Results of routine laboratory tests were normal.
After initial assessment, he was diagnosed as major depression with psychotic symptoms. NT (50 mg/day), lorazepam (1 mg/day), and risperidone (2 mg/day) were initially prescribed. Supportive psychotherapy was also provided. The patient's dose of NT was then increased gradually to 100 mg/day, administered on a divided-dose schedule. Determination of plasma NT level was performed by high-performance liquid chromatography using Hewlett Packard 1090 system (Hewlett Packard GmbH, Waldbronn, Germany). The plasma concentration of NT after 6 days of treatment with 100 mg of daily dose was 181.4 ng/mL (recommended therapeutic range: 50-150 ng/mL).
However, in an attempt to achieve further clinical improvement, the NT dosage was increased to 150 mg/day over 20 days, which resulted in plasma concentrations of 470.6 ng/mL after 6 days. He reported experiencing dry mouth, constipation, and dizziness. Because of this unexpectedly high drug level and worsening of anticholinergic side effects, the patient's dosage was reduced to 100 mg/day. The following measurements indicate that NT concentrations were reduced (198.7-222.7 ng/mL) but still remained higher than the recommended therapeutic range. The patient was discharged after 1 month of hospitalization with a maintenance dose of 100 mg/day.
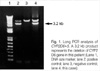
This patient underwent CYP2D6 genotyping test after informed consent. CYP2D6 gene was amplified by long PCR, to analyze all 9 exons of CYP2D6 gene (8). All PCR products were sequenced using ABI PRISIM BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, U.S.A.) and an ABI Prism 3100 Genetic Analyzer (Applied Biosystems). CYP2D6*5 allele was detected using long PCR as described by Hersberger et al. (9). A fragment specific for deletion of CYP2D6 gene was obtained with the forward primer specific for exon 9 of CYP2D7 and the reverse primer specific for the 3'flanking region of CYP2D6. The patient was found to have CYP2D6*5/*10B, which results in low CYP2D6 activity (Fig. 1, 2).
He has been continuing supportive psychotherapy at the outpatient clinic monthly and remains improved, even though his NT dose has been reduced to 50 mg/day.
Plasma concentrations of TCAs have been found to correlate well with drug responses (1). The recommended therapeutic plasma levels of NT are between 50 ng/mL and 150 ng/mL (1, 2). Levels above this range have been associated with an increased incidence of side effects. Generally, medications of NT begin at low doses (50-75 mg/day). The dose is then increased gradually to an average dose of 150 mg/day until the optimal therapeutic range is reached.
However, steady-state concentrations in plasma observed in the course of treatment with TCAs are poorly related to the administered dose, and values in patients who were given similar doses of TCAs can vary from 10- to 35- fold (1-4). Patients on the same dose can develop sub-optimal, therapeutic or toxic concentrations depending on their clearance rate (1). Interindividual variation in the metabolism of NT is one of the important determinants for plasma levels or adverse effects of the drugs.
CYP2D6, which is involved in the hepatic metabolism of NT, contributes to broad interpatient variability. Previous reports have suggested that the steady-state concentrations of the drugs were genetically controlled (4-7). Subjects with more than one inactive CYP2D6 allele demonstrate poor metabolizer phenotype and develop high plasma NT concentrations (5, 7). Accordingly, poor metabolizers of CYP2D6 are more prone to NT overdose.
About 5-10% of Caucasians and 1% of Asians are poor metabolizers with regard to CYP2D6 substrates (4, 10). The frequency of non-functional allele CYP2D6*5, with deletion of the entire CYP2D6 gene, is 4-6% of the populations (10, 11). CYP2D6*10 is the most prevalent allele in Asians and its frequency ranges from 38-70% (10-12). CYP2D6*10 allele is associated with significantly reduced metabolic activity, approximately 60% of normal activity (10).
In this report, the patient revealed decreased CYP2D6 enzyme activity with the genotype of CYP2D6*5/*10B. Therefore, he attained drug concentrations above the therapeutic range at the standard doses and might be at a higher risk of developing adverse reactions. This patient could have taken appropriate therapeutic changes earlier and maintained his psychiatric gains at a lower dose, if he had been genotyped at the beginning of his NT therapy.
Treatment with two or more drugs metabolized by the same CYP enzyme may result in interactions such as competition of the enzyme and decreased rates of metabolism (4). Shim et al. (13) reported that paroxetine, a potent CYP2D6 inhibitor, increased the blood levels of tricyclic antidepressant in 10 Korean schizophrenic patients. As risperidone is a weak inhibitor of CYP2D6 enzyme and its metabolite is pharmacologically equipotent to the parent drug, drug interactions related to polymorphic metabolism would be expected to have limited clinical significance (4, 14).
Clinicians are used to adjusting the dose based on clinical response and plasma drug concentrations. Additional CYP2D6 genotyping can serve as an important guide for more rapid dosage adjustment and prediction of drug response in each individual. CYP2D6 genotyping is recommended to complement therapeutic drug monitoring especially when the unusual metabolic capacity of NT is suspected. Moreover, due to the potential seriousness and cost of adverse effect including cardiac toxicity (15), we can emphasize the importance of CYP2D6 genotyping in dosage adjustment of NT.
Figures and Tables
References
1. Preskorn SH. Pharmacokinetics of antidepressants: why and how they are relevant to treatment. J Clin Psychiatry. 1993. 54:14–34.
2. Linder MW, Keck PE Jr. Standards of laboratory practice: antidepressant drug monitoring. Clin Chem. 1998. 44:1073–1084.
3. Morita S, Shimoda K, Someya T, Yoshimura Y, Kamijima K, Kato N. Steady state plasma levels of nortriptyline and its hydroxylated metabolites in Japanese patients: Impact of CYP2D6 genotype on the hydroxylation of nortriptyline. J Clin Psychopharmacol. 2000. 20:141–149.
4. Bertilsson L, Dahl ML, Dalen P, Al-Shurbaji A. Molecular genetics of CYP2D6: Clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002. 53:111–122.

5. Dahl ML, Bertilsson L, Nordin C. Steady-state plasma levels of nortriptyline and its 10-hydroxy metabolite: relationship to the CYP2D6 genotype. Psychopharmacology. 1996. 123:315–319.
6. Dalen P, Dahl ML, Ruiz ML, Nordin J, Bertilsson L. 10-hydroxylation of nortriptyline in white persons with 0, 1, 2, 3 and 13 functional CYP2D6 genes. Clin Pharmacol Ther. 1998. 63:444–452.

7. Bertilsson L, Mellstrom B, Sjokvist F, Martension B, Asberg M. Slow hydroxylation of nortriptyline and concomitant poor debrisoquine hydroxylation: clinical implications: letter. Lancet. 1981. 1:560–561.
8. Kimura S, Umeno M, Skoda RC, Meyer UA, Gonzalez FJ. The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am J Hum Genet. 1989. 45:889–894.
9. Hersberger M, Marti-Jaun J, Rentsch K, Hanseler E. Rapid detection of the CYP2D6*3, CYP2D6*4, CYP2D6*6 alleles by tetra-primer PCR and of the CYP2D6*5 allele by multiplex long PCR. Clin Chem. 2000. 46:1072–1077.
10. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002. 3:229–243.

11. Kubota T, Yamaura Y, Ohkawa N, Hara H, Chiba K. Frequencies of CYP2D6 mutant alleles in a normal Japanese population and metabolic activity of dextromethorphan o-demethylation in different CYP2D6 genotypes. Br J Clin Pharmacol. 2000. 50:31–34.
12. Roh HK, Dahl ML, Johansson I, Ingelman-Sundberg M, Cha YN, Bertilsson L. Debrisoquine and S-mephenytoin hydroxylation phenotypes and genotypes in a Korean population. Pharmacogenetics. 1996. 6:441–447.

13. Shim JC, Gong B, Park JH, Yoon YR, Sin JG, Kim JI, Ahn DS, Kim YK, Cha IJ, Kim YH. Combined therapy of paroxetine and tricyclic antidepressant in depression of schizophrenic patients. J Korean Neuropsychiatr Assoc. 1997. 36:732–741.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download