Abstract
The role of lung mast cells in exercise-induced asthma (EIA) is controversial. To investigate whether the skin mast cell releasability is increased after exercise in EIA, 49 young atopic men with or without asthma took part in a free-running test for 6 min and were given skin prick tests using morphine, a mast cell secretagogue, before and after the exercise. The mean diameters of the wheal induced by morphine in patients with EIA were not significantly different from those in patients without EIA before exercise, although the baseline lung function was significantly lower and the airway hyperresponsiveness, the peripheral blood eosinophil count, and the size of the wheal in response to Dermatophagoides pteronyssinus were significantly higher in patients with EIA. However, the differences of the morphine-induced wheal diameter between patients with EIA and those without EIA became significant at 120 min after exercise (p<0.05), while the responses to histamine were not significantly different. These results suggest that exercise increases the releasability of skin mast cells in EIA patients whose asthma/allergy are relatively severe.
Although the exact mechanisms underlying exercise-induced asthma (EIA) are unknown, it has been suggested that rapid breathing may cause the evaporation of mucosal surface water and an increase in osmolarity, which then results in mast cell degranulation and the constriction of airway smooth muscle (1, 2). Several investigators (3, 4) have shown evidence of mast cell activation following exercise in asthmatics, while others (5) have failed to show any relationship between the degranulation of mast cells and EIA.
It has been shown that atopic subjects had increased releasability of mast cells in response to nonimmunologic stimulation, such as morphine (6, 7). In addition, exercise-induced histamine release has been demonstrated in exercise-induced urticaria and anaphylaxis (8). Moreover, there was a marked increase in the wheal response to compound 48/80 (a cutaneous mast cell secretagogue) and not to histamine after a combination of food and exercise challenge in patients with food-associated exercise-induced urticaria-angioedema (9).
Because atopic individuals have an increased releasability of mast cells and some atopic patients show manifestations related with mast cell mediators following exercise, atopic patients with EIA also might have an increased releasability of mast cells in both the lung and the skin. However, no study has investigated this. Therefore, this study investigated, to infer the role of the mast cell mediators in EIA, whether the wheal response to a mast cell degranulating agent is increased in EIA and whether the response is increased with exercise.
Thirty-eight consecutive patients with mild asthma who visited Chonnam National University Hospital, Gwangju, Korea, and 11 normal control subjects were enrolled in this study. They were young men (18-23 yr old) who were atopic, as demonstrated by at least one positive skin prick test to common inhalant allergens. All of the patients were those who required a medical certificate for asthma in order to be exempted from obligatory military service. Normal control subjects were students of Chonnam National University Medical School who were atopic, but without any history of atopic diseases including atopic dermatitis. All subjects had neither dermographism nor other symptomatic atopic skin diseases. Asthma and its severity were defined according to the Global Strategy for Asthma Management and Prevention (10). The Institutional Review Board of Chonnam National University Hospital approved the study. All the subjects were informed of the experimental procedures and provided written informed consent.
A methacholine (MCh) bronchoprovocation test was performed in the patients with asthma. On the next day, a free-running test was carried out. Skin prick tests were done immediately before and 30-60 and 120 min after the exercise.
Lung function was measured with a spirometer (Spiro Analyzer ST-250, Fukuda Sangyo, Tokyo, Japan). Each subject performed the tests using techniques that meet the standards developed by the American Thoracic Society (11). The largest forced expiratory volume in one second (FEV1) from more than three acceptable curves was selected as the representative value. MCh bronchoprovocation tests were performed using a standardized tidal breathing method (12). The provocative concentration of MCh resulting in a 20% fall in FEV1 (PC20) was calculated by linear interpolation of the log-dose-response curve.
The free-running test was performed for 6 min, as described previously (13). Briefly, during the first 2-3 min of exercise, the subjects accelerated, raising their pulse rate to 85% of their predicted maximum (calculated as 220 min age in years). For the remaining time, the subjects ran without changing their velocity. FEV1 was measured immediately before and 1, 3, 5, 7, 10, 15, 20, 30, 45, and 60 min after exercise. The maximal change in FEV1 was calculated as 100%×[(preexercise FEV1 minus lowest post-exercise FEV1)/pre-exercise FEV1]. Positive EIA was defined as a 15% or more reduction in the post-exercise FEV1 as compared with the pre-exercise FEV1.
Skin prick tests were carried out with 15 common aeroallergens and morphine sulfate (1 mg/mL) on the back immediately before exercise, along with histamine dihydrochloride (1 mg/mL), and saline (0.9%) solutions as positive and negative controls, respectively. The allergens were as follows: elder, birch, hazel, Bermuda grass, timothy, mugwort, and ragweed pollens, cat and dog dander, the mites Dermatophagoides pteronyssinus (Der p) and D. farinae, cockroach, and Alternaria, Aspergillus, and Penicillium (Bencard, Brantford, England). The skin prick tests with Der p, morphine, histamine, and saline were repeated 30-60 min and 120 min after the exercise. The longest and perpendicular diameters of each wheal were measured with a millimeter ruler 15 min after the prick, and the arithmetic mean of the recorded measurements was used as the representative value. The positive response to morphine was defined as the wheal size to morphine ≥2 mm. According to the ratio of the size of the allergen-induced wheal to that elicited by the histamine solution (A/H ratio), reactivity was graded as follows: 25-49%: 1+; 50-99%: 2+; 100-199%: 3+; ≥200%: 4+. The atopy score was defined as the sum of the grades to all 15 allergens. The subject with ≥2+ to at least one allergen was regarded as atopic.
PC20 values were log transformed before the statistical analysis, and presented as geometric mean values. The results were expressed as the mean±SEM. The data were compared using Student's t-test unpaired and paired for intergroup and intragroup comparisons, respectively, and χ2 tests. Mann-Whitney U test was used to reexamine the intergroup comparison. Associations between variables were examined using the Pearson correlation coefficient. p<0.05 was regarded as statistically significant.
The characteristics of patients with or without EIA compared to those of normal controls are presented in Table 1. Twenty-four (63.2%) patients had EIA. There were no significant differences between the asthmatics and controls in terms of age, atopy score, or the size of wheal to Der p. However, the baseline FEV1 was significantly lower in asthmatics (p<0.05). There were no significant differences between patients with EIA and those without EIA in terms of age, asthma duration, FEV1, or atopy score. However, the MCh-PC20 was significantly lower (p<0.001) and the peripheral blood eosinophil count (p<0.01) and size of the wheal in response to Der p (p<0.05) were significantly higher in patients with EIA than in those without EIA. The maximal fall in FEV1 following exercise was significantly related to the MCh-PC20 (r=-0.661, p<0.001), the peripheral blood eosinophil count (r=0.551, p<0.001), and the size of the wheal in response to Der p (r=0.344, p<0.05) in the patients with asthma (Fig. 1).
All the subjects tolerated the exercise test well and none developed urticaria. The mean maximal fall in FEV1 in the patients with EIA was 32.8±2.5%, with the peak 5 min after completing the exercise. By contrast, the FEV1 values remained stable in the patients without EIA and the normal controls.
The post-exercise skin prick test responses to histamine, morphine, and Der p as compared to the pre-exercise levels are presented in Table 2. The pre-exercise wheal sizes in the patients were not significantly different from the controls. However, the post-exercise size in response to morphine was significantly larger in the patients with EIA than in the controls (at 30-60 min: p<0.001; at 120 min: p<0.001), while there were no significant differences for histamine. Particularly, the post-exercise response to morphine was significantly larger in the patients with EIA than in the patients without EIA at 120 min (p<0.05). The response to morphine was also significantly larger in patients without EIA than in the controls 30-60 min after the exercise (p<0.05). The comparisons using Mann-Whitney U test also showed a similar result.
Similarly, the pre-exercise positive rates to morphine were not significantly different from the controls (EIA (+): 25.0%, EIA (-): 35.7%, normal: 9.1%; Fig. 2). However, the post-exercise positive rate to morphine was significantly higher in the patients with EIA than in the controls (45.8% vs. 0% at 30-60 min: p<0.01; 57.9% vs. 0% at 120 min: p<0.01). Particularly, the post-exercise positive rate to morphine was significantly higher in the patients with EIA than in the patients without EIA at 120 min (57.9% vs. 16.7%, p<0.05).
Although the post-exercise size in response to morphine tended to be larger than the pre-exercise value, the difference was not reached to the statistically significant level (EIA (+): p=0.25, p=0.29; EIA (-): p=0.99, p=0.32; controls: p=0.34, p=0.34 at 30-60 min, at 120 min, respectively). Similarly, the post-exercise positive rate to morphine tended to be higher than the pre-exercise value without a statistically significant difference. And the post-exercise change in size to morphine in the patients with EIA tended to be higher than those in the other groups (EIA (-): p=0.51, p=0.15; controls: p=0.14, p=0.18 at 30-60 min, at 120 min, respectively). The wheals in response to Der p were significantly larger in the patients with EIA than in those without EIA before and after exercise (p<0.05, respectively).
This study showed that exercise increased the skin responses to morphine, but not to histamine, in asthmatic patients with EIA. It has been reported that µ-receptor agonists, such as morphine sulfate, codeine phosphate, and meperidine hydrochloride, elicit immediate positive skin reactions as a consequence of mast cell degranulation (14). Therefore, these results suggest that exercise increases the mediator releasability of skin mast cells, but not the responsiveness of the skin vessels in patients with EIA. To our knowledge, this is the first study to show that EIA is associated with the increased releasability of skin mast cells.
Since FEV1 and MCh-PC20 were significantly lower and the peripheral blood eosinophil count and the size of wheal in response to Der p were significantly higher in patients with EIA than in those without EIA in this study, the underlying allergic diseases were apparently more severe in patients with EIA, and therefore more susceptible to nonspecific stimuli. Asthma is an airway disease that is characterized by chronic inflammation, which causes an associated airway hyperresponsiveness to various stimuli. There are correlations between the numbers of mast cells, eosinophils, and neutrophils and the degree of airway hyperresponsiveness (15). Although there is some controversy over the relationship between the severity of EIA and the clinical severity of asthma (16), in this study the maximal fall in FEV1 was related to MCh-PC20, the peripheral blood eosinophil count, and the size of the wheal in response to Der p in the patients with asthma, which is consistent with previous studies showing a relationship between exercise-induced bronchospasm and the blood eosinophil count (17) or the size of the wheal in response to Der p (13). Therefore, EIA might be due to underlying severe allergic airway disease in patients with asthma.
A similar exercise-induced phenomenon may also occur in the skin of patients with EIA. Although the predominant type of mast cells found in the lung (MCT) differs from that in the skin (MCTC), and allergic airway diseases are not always accompanied by allergic skin diseases, we speculate that in patients with EIA the skin also shows anatomical or functional changes that make it more vulnerable to nonspecific stimuli. One such alteration would be the increased releasability of skin mast cells.
The increased releasability of mast cells in response to non-immunologic stimulation has been demonstrated in atopic subjects (6, 7). However, atopy alone (the control subjects were also atopic) and increased releasability of mast cells alone may be insufficient, and other factors, including target organ sensitivity, may be needed to elicit reactions to exercise in the airways or skin. In addition, exercise alone did not induce urticaria and the skin response to morphine alone was not increased significantly, even in the patients with EIA. However, the combination of both exercise and morphine resulted in a significantly larger/higher urticarial response in the asthmatics with EIA compared with that in those without EIA. This additive effect of exercise to other stimuli, such as methacholine (18) or compound 48/80 (a cutaneous mast cell degranulating agent) (9) on the skin response has also been reported in patients with urticaria.
Exercise increased the mast cell releasability. Exercise did not increase skin reactivity to Der p, suggesting that mast cell activation via IgE receptors differs from that via non-IgE receptors. A similar finding has been reported in a case of exercise-induced anaphylaxis to food, in which the skin reactivity to food did not increase following exercise, while the codeine-induced wheal size did increase (19). And, in the reverse order to the effect of exercise on the subsequent skin response to morphine, it has been reported that prior methacholine skin testing caused local urticaria at the skin test site following an exercise test in some patients with cholinergic urticaria (18). Therefore, the combination of exercise and morphine might increase the skin response nonspecifically in patients with severe underlying allergic disease, such as the patients with EIA in this study.
These findings suggest that increased mast cell releasability of the lung also plays a role in EIA, at least for a short period following exercise. However, there is some controversy over the role of mast cells in EIA, and the type of the lung mast cells differs from that of the skin mast cells. Therefore, it would be premature to conclude that the same events that occurred in the skin might occur also in the lung, and further studies are necessary to resolve this.
Taken together, exercise increased the skin responses to morphine, but not to histamine, in asthmatic patients with EIA. Because the underlying allergic diseases were apparently more severe in patients with EIA, their skin would be more vulnerable to nonspecific stimuli, such as exercise and morphine. Likewise, increased mast cell releasability of the lung also would play a role in EIA.
Figures and Tables
Fig. 1
Relationship between the markers of severity of asthma/allergy (X-axes) and the degree of exercise-induced airway obstruction (Y-axes). FEV1, Forced expiratory volume in one second; PC20, Provocative concentration of methacholine resulting in a 20% fall in FEV1.
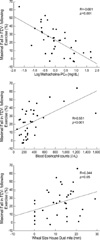
Fig. 2
The positive response rate to morphine skin prick test (≥2 mm in wheal size) in patients with exercise-induced asthma (EIA) (diamonds, n=24), patients without EIA (squares, n=14), and normal controls (triangles, n=11) after exercise. Skin prick tests were performed before and at 30-60 min and 120 min after exercise. *p<0.01 compared with normal controls, †p<0.05 compared with patients without EIA.

Table 1
Characteristics of subjectCharacteristics of subject
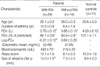
The results were expressed as mean±SEM. EIA, exercise-induced asthma; FEV1, forced expiratory volume in one second; Log-PC20, the logarithmically transformed provocative concentration of methacholine resulting in 20% fall in FEV1; Atopy score, the number of "+" from skin reactions to 15 allergens, in which each reaction was graded from 1+ to 4+. Der p, Dermatophagoides pteronyssinus. *p<0.05, †p<0.01, and ‡p<0.001 compared with normal controls. §p<0.05, ∥p<0.01, and ¶p<0.001 compared with patients without EIA.
References
1. Anderson SD. Is there a unifying hypothesis for exercise-induced asthma? J Allergy Clin Immunol. 1984. 73:660–665.

2. Sheppard D, Eschenbacher WL. Respiratory water loss as a stimulus to exercise-induced bronchoconstriction. J Allergy Clin Immunol. 1984. 73:640–642.

3. Lee TH, Brown MJ, Nagy L, Causon R, Walport MJ, Kay AB. Exercise-induced release of histamine and neutrophil chemotactic factor in atopic asthmatics. J Allergy Clin Immunol. 1982. 70:73–81.

4. O'Sullivan S, Roquet A, Dahlen B, Larsen F, Eklund A, Kumlin M, O'Byrne PM, Dahlen SE. Evidence for mast cell activation during exercise-induced bronchoconstriction. Eur Respir J. 1998. 12:345–350.
5. Broide DH, Eisman S, Ramsdell JW, Ferguson P, Schwartz LB, Wasserman SI. Airway levels of mast cell-derived mediators in exercise-induced asthma. Am Rev Respir Dis. 1990. 141:563–568.

6. Turner CR, Darowski MJ, Sampson HA, Spannhake EW, Hirshman CA. Dermal mast cell releasability and end organ responsiveness in atopic and nonatopic dogs. J Allergy Clin Immunol. 1989. 83:643–648.

7. Choi IS, Park SC, Kang KW. Clinical usefulness of morphine skin prick test in diagnosis of allergic diseases. J Asthma Allergy Clin Immunol. 1999. 19:476–483.
8. Casale TB, Keahey TM, Kaliner M. Exercise-induced anaphylactic syndromes. Insights into diagnostic and pathophysiologic features. JAMA. 1986. 255:2049–2053.

9. Kivity S, Sneh E, Greif J, Topilsky M, Mekori YA. The effect of food and exercise on the skin response to compound 48/80 in patients with food-associated exercise-induced urticaria-angioedema. J Allergy Clin Immunol. 1988. 81:1155–1158.

10. National Institutes of Health. NIH NHLBI Revised 2002. NIH Publication No. 02-3659. Global strategy for asthma management and prevention. 2002.
11. American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995. 152:1107–1136.
12. Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness: standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur Respir J Suppl. 1993. 16:Suppl. 53–83.
13. Koh YI, Choi IS, Lim H. Atopy may be related to exercise-induced bronchospasm in asthma. Clin Exp Allergy. 2002. 32:532–536.

14. Casale TB, Bowman S, Kaliner M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: evidence for opiate and nonopiate receptor participation. J Allergy Clin Immunol. 1984. 73:775–781.

15. O'Byrne PM. Middleton E, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, Busse WW, editors. Airway hyperresponsiveness. Allergy principles & practice. 1998. 5th ed. Toronto: Mosby;859–866.
16. Godfrey S. McFadden ER, editor. Clincal and physiological features. Exercise-Induced Asthma. 1999. New York: Marcel Dekker, Inc.;11–45.
17. Koh YI, Choi IS. Blood eosinophil counts for the prediction of the severity of exercise-induced bronchospasm in asthma. Respir Med. 2002. 96:120–125.

18. Kiistala R, Kiistala U. Local cholinergic urticaria at methacholine test site. Acta Derm Venereol. 1997. 77:84–85.
19. Lin RY, Barnard M. Skin testing with food, codeine, and histamine in exercise-induced anaphylaxis. Ann Allergy. 1993. 70:475–478.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download