Abstract
188Re(Rhenium) is easily obtained from an in-house 188W/188Re generator that is similar to the current 99Mo/99mTc generator, making it very convenient for clinical use. This characteristic makes this radionuclide a promising candidate as a therapeutic agent. Polyethylenimine (PEI) is a cationic polymer and has been used as a gene delivery vector. Positively charged materials interact with cellular blood components, vascular endothelium, and plasma proteins. In this study, the authors investigated whether intratumoral injection of 188Re labeled transferrin (Tf)-PEI conjugates exert the effect of radionuclide therapy against the tumor cells. When the diameters of the Ramos lymphoma (human Burkitt's lymphoma) xenografted tumors reached approximately 1 cm, 3 kinds of 188Re bound compounds (HYNIC-PEI-Tf, HYNIC-PEI, 188Re perrhenate) were injected directly into the tumors. There were increases in the retention of 188Re inside the tumor when PEI was incorporated with 188Re compared to the use of free 188Re. The 188Re HYNIC-Tf-PEI showed the most retention inside the tumor (retention rate=approximately 97%). H&E stain of isolated tumor tissues showed that 188Re labeled HYNIC-PEI-Tf caused extensive tumor necrosis. These results support 188Re HYNIC-PEI-Tf as being a useful radiopharmaceutical agent to treat tumors when delievered by intratumoral injection.
188Re (Rhenium) is a short-lived beta emitting radionuclide (physical half-life=17 hr, Emax=2.11 MeV). It has an average beta particle penetration of 3.3 mm (maximum=10.8), providing a tightly circumscribed region of high-energy deposition with little damage to the adjacent cells and organs. In addition, 188Re is easily obtained from an in-house 188W/188Re generator similar to the current 99Mo/99mTc generator, which makes it convenient for clinical use (1-4). These characteristics make this radionuclide a promising candidate as a therapeutic agent.
Polyethylenimine (PEI) is a cationic polymer that has recently appeared as a possible alternative to viral and liposomal routes for gene delivery (5). PEI has been studied as a chelating agent to remove heavy metal ions (6, 7). Because PEI contains primary, secondary, and tertiary amines, PEI derivatives can be chelated with a radionuclide such as 188Re. PEI can also be coupled to ligands such as galactose or transferrin (Tf) for the purpose of targeting specific organ or tumor cells (8-12). Therefore, ligand binding PEI derivatives labeled with 188Re may be used as a novel radiopharmaceutical in targeting therapy for tumor treatment.
Use of Tf conjugates for site-specific drug delivery and gene delivery to tumor cells has been studied via intravenous injection (13, 14). Xu et al. reported that an intratumoral injection using the Tf-liposome system showed a higher number of transfected tumor cells in vivo when compared with transfection by liposome alone (15). These results indicate that irrespective of the route of injection, Tf conjugates specifically bind to Tf receptors (Tf-R) present in the tumor cell membrane. These complexes then enter the cells by receptor-mediated endocytosis. This study investigated whether intratumoral injections of 188Re labeled Tf-PEI conjugates exert the full effect of radionuclide therapy against the tumor cells.
Tf-PEI (branched PEI, 25 kDa) conjugates were synthesized as described by Kircheis and coworkers (16). Branched PEI was purchased from Aldrich (WI, U.S.A.). Briefly, human apo-transferrin in 30 mM sodium acetate buffer (pH 5.0) was subjected to gel filtration on a Sephadex G-25 superfine column (Pharmacia, Uppsala, Sweden). The resulting solution was cooled to 0℃ and sodium periodate (in 30 mM pH 5 sodium acetate buffer) were added. The mixture was kept in an ice bath in the dark for 90 min and promptly added to PEI solution and mixed at room temperature. After 30 min, four portions of sodium cyanoborohydride were added at 1 hr intervals. After 18 hr, 3 M sodium chloride was added. The reaction mixture was loaded on a cation-exchange column, Macro-Prep high S HR 10/10 (Bio-Rad, Hercules, CA, U.S.A.) and was fractionated with a salt gradient from 0.5 M to 3.0 M sodium chloride in 20 mM HEPES (pH 7.3). A 3 molar excess of dissolved succinimidyl 6-hydrazino nicotinate hydrochloride (HYNIC) (5 mol% of PEI amino group) in 30 mM dimethyl-formamide (DMF) was added dropwise to a stirred solution of Tf-PEI conjugates. The solution was stirred gently for 24 hr at 4℃ protected from light. This was followed by dialysis against HBS (pH 7.3, 150 mM sodium chloride, 20 mM HEPES) at 4℃ (six buffer changes for 72 hr). The iron incorporation was performed by addition of 1.25 µL 10 mM iron (III) citrate buffer per mg transferrin content. The conjugates were divided into convenient small aliquots and kept at -20℃.
188Re-perrhenate was obtained in 20 mL of normal saline from 188W/188Re generator (Shanghai Ke Xing Pharmaceuticals, Shanghai, China). The labeling process was carried out in the presence of stannous chloride dehydrate. 188Re sodium perrhenate eluate (370 MBq) in 0.9% normal saline was mixed in a vial with 10 mg of ascorbic acid, 40 mg of SnCl2 (Sigma, U.S.A.), and 500 µL of HYNIC-PEI-Tf (1 µg/µL) or HYNIC-PEI. Then, the vial was reacted at 37℃ for 1 hr. The labeling yield was determined by ITLC-SG (Gelman Science, Ann Arbor, MI, U.S.A.) using acetone as the mobile phase.
To generate tumors, three 5- to 6-week-old female BALB/c nude mice (Orient Co. Ltd., Seoul, Korea) were injected subcutaneously in the left thigh with Ramos cells (ATCC CRL 1596, human Burkitt's lymphoma, 5×106 cells/100 µL). Tumor size was measured using a vernier caliper across its longest and after the diameters were reached about 1 to 1.5 cm, 3 kinds of 188Re labeled compounds (300 µCi of HYNIC-PEI-Tf, HYNIC-PEI, and free 188Re) were injected directly three times into the tumors, respectively.
Static images were acquired with a gamma camera (Vertex, ADAC, Milpitas, CA, U.S.A.) equipped with a low energy, pin-hole collimator, and with a 40% window centered over the 155-keV photopeak. Gamma images were achieved from 10 min to 12 hr after injection to certifying intratumoral retention of 188Re HYNIC-PEI-Tf (11.1 MBq/mouse) and 188Re HYNIC-PEI in comparison with that applied with same dose of 188Re perrhenate.
Histologic evaluation with H&E staining was performed 2 days after injection. The frozen sections were washed with tap water for 5 min, immersed in hematoxylin for 2 min and checked for complete staining in tap water. Eosin staining was carried out for 3 min. Sections were dehydrated through a graded series of alcohol (70 to 100% ethanol, 3 min each), cleared in xylene, cover-slipped and observed with a light microscope.
Region of interest (ROI) were drawn manually on 10 min image around the tumor and whole body. ROIs of the same size and shape were applied to 2 hr and 12 hr images. Retention rates of 3 radiotracers were calculated. A half-life for 188Re HYNIC-PEI-Tf was also calculated in the form of effective half-life and the residence time was defined as half-life/ln 2. Tumor self-radiation S-value of 188Re HYNIC-PEI-Tf was obtained using the Nodule Module in MIRDOSE3.1 software (Oak Ridge Associated University) (17). From the calculated S-values and residence time in the mouse model, the dose of 188Re HYNIC-PEI-Tf to obtain 100 Gy of tumor irradiation was calculated.
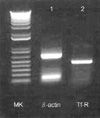
Total RNA isolated from Ramos cells with Trizol reagent (Invitrogen, U.S.A.) was used for RT-PCR. First-strand cDNA synthesis was performed at 42℃ for 50 min, followed by 70℃ for 15 min using Superscript II reverse transcriptase and oligo (dT)10 as the primer. The cDNA was amplified using the following primers: Tf-R F 5'CTCACTTTAGACAATGCTGC 3'; Tf-R R 5'CTCATGACACGATCATTGAG 3'. A pair of primers specific to β-actin (F 5'-TGACGGGGTCACCCACACTGTGCCCATC TA-3'; R 5'-CTAGAAGCATTTGCGGTGGACGATGCAGGG-3') was used as a control. PCR was performed in a DNA thermal cycler using 94℃ melting, 45℃ annealing, and 72℃ extension temperatures for 33 cycles. The PCR products were loaded on agarose gel (1%) containing ethidiume bromide and electrophoresis was performed at 100 V for 20 min.
The labeled 188Re HYNIC-PEI-Tf or HYNIC-PEI remained localized at the origin of injection and radiochemical purities of these labeled compounds at 15 min and 1 hr were 97% and 80%, respectively.
Fig. 1A, B showed that when PEI was incorporated with 188Re, the retention of 188Re inside the tumor was increased compared with free 188Re. The 188Re HYNIC-PEI-Tf mostly remained in the tumor and showed a higher retention rate than 188Re HYNIC-PEI (approximately 97% vs. 85%). 188Re HYNIC-PEI released from the tumor accumulated in the liver and lungs. Fig. 1C showed that free 188Re escaped from the tumor over time and that there was no remaining 188Re 12 hr after injection. The 188Re released accumulated in the stomach, thyroid, and bladder in much the same way that 99mTc pertechnetate did.
Effective half-life of 188Re HYNIC-PEI-Tf was assumed to be same to the physical half-life. The caculated residence time of this conjugate was 24.5 hr. The nodular self-dose S-value was estimated as 0.343 mGy/MBq·h. using MIRDOSE3.1. The radioactivity required for a target irradiation dose of 100 Gy for 0.97 to 1.23 cm tumor was calculated 6.4 to 11.9 MBq for 188Re HYNIC-PEI-Tf from this S-value.
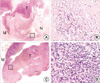
Representative hematoxylin and eosin-stained sections of the isolated tumors injected with 188Re HYNIC-PEI-Tf and 188Re perrhenate were shown in Fig. 2. In the tumor injected with 188Re HYNIC-PEI-Tf, histological changes were found in contrast with 188Re perrhenate. 188Re HYNIC-PEI-Tf made extensive central necrosis inside the tumor and remained the small portion of viable tumor tissue around the tumor mass.
The result of RT-PCR represented the Ramos Burkitt's lymphoma cells had Tf-R mRNA and the possibility of expression of Tf-R protein on the cell membrane (Fig. 3).
Our preliminary results show that intratumoral injection with 188Re HYNIC-PEI-Tf caused extensive necrosis in the xenografted tumor without a significant leakage of radioactive compound. Although PEI could chelate with 99mTc or 188Re because of the secondary or tertiary amine groups in this polymer, in this study HYNIC was introduced as a biconjugate for higher and safer labeling.
188Re has not only strong potential for therapeutic use but also excellent imaging characteristics because of its β energy (2.1 MeV), its short physical half-life (17 hr) and its 155 keV γ-ray emission; often used for dosimetric and imaging purposes. Therefore many researchers have used 188Re in a variety of fields such as intravascular radiation, radioimmunotherapy using monoclonal antibodies, and metastatic bone lesions (2-4). Radiotherapy using 188Re needs novel therapeutic strategies, which can facilitate an increase in the intratumoral uptake or a retention and reduction in its systemic levels. Radiotherapy with intratumorally-injected radiopharmaceuticals is a promising approach to some kinds of tumor such as head and neck cancers because it offers the potential to localize the radiation inside the tumor. Because potential leakage of therapeutic radionuclide causes serious problems in the site of accumulation, there is a need to minimize leakage of the injected compound. Intratumoral injection of 188Re HYNIC-PEI-Tf resulted in the highest retention in the tumor mass indicating this approach had strong potential for the treatment of solid tumors.
PEI is a cationic polymer and has been used as a gene delivery vector. Positively charged materials interact with cellular blood components, vascular endothelium, and plasma proteins when they are injected systemically (18, 19). In this study, 188Re HYNIC-PEI leaked out from injected site showed the liver and lung activity, this might be explained that positive surface charge of PEI led to interactions with lung and hepatic endothelium. Until now, there have been few reports about intratumoral injection using 188Re labeled cationic polymer. Therefore, despite of incomplete and preliminary data, our result is thought to be introduced the new, useful compound to the field of radionulide therapy.
High retention of 188Re HYNIC-PEI in the tumor demonstrates that the intratumoral injections of positively charged radioactive conjugates bind to tumor cells adjacent to the injection sites. Based on the fact that Tf-PEI derivatives/DNA complexes have been known to deliver the gene efficiently through the Tf-Tf receptor system, in the intratumoral approach of 188Re HYNIC-PEI-Tf, Tf-Tf receptor mediated tumoral uptake is thought to play an additional role for higher retention of radiocompound in the tumor than that of 188Re HYNIC-PEI. We certified the existence of Tf-receptor mRNA through RT-PCR method in the Ramos lymphoma cell line. Another role of Tf conjugate as a ligand may be that Tf enlarged the size of 188Re labeled polymer because the molecular weight of Tf is relatively heavy, about 80 kDa.
As shown in Fig. 1, Fig. 2, high retention of 188Re labeled cationic polymers in the tumor were not only inspected through nuclear imaging of good quality because of appropriate gamma energy for imaging, but also predicted extensive necrosis around the sites of injection. We verified that injected and retained dose, 11.1 MBq, of 188Re HYNIC-PEI-Tf was enough to cause the necrosis to approximately 1cm-sized tumor through calculated results for dosimetry using the Nodule Module in MIRDOSE3.1 software.
In conclusion, the results of this preliminary study show that 188Re labeled HYNIC-PEI-Tf can be a useful radiopharmaceutical agent to treat solid tumors when delivered by intratumoral injection.
Figures and Tables
Fig. 1
Gamma images of Ramos lymphoma xenografted nude mice 10 min, 2 hr, and 12 hr after intratumoral injection. (A) Of three kinds of 188Re conjugates, 188Re HYNIC-PEI-Tf showed the highest retention rate inside the tumor and relatively no leakage from the tumor when radionuclides were injected intratumorally. (B) With time, 188Re HYNIC-PEI escaped from the tumor with some extent (approximately 15%) and accumulated into the lung and liver.
(A) 188Re HYNIC-PEI-Tf injected mouse, (B) 188Re HYNIC-PEI injected mouse, (C) 188Re perrhenate injected mouse.
L, liver; T, tumor; ch, chest; th, thyroid; S, stomach; B, bladder.
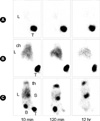
Fig. 2
Hematoxylin-eosin staining obtained 48 hr after intratumoral injection. (A, B) Histological findings (H&E, original magnification ×10, ×200) demonstrate that wide central necrosis with peripheral viable cells is shown when 188Re HYNIC-PEI-Tf is injected, but (C, D, H&E ×10, ×200) no significant necrosis when 188Re perrhenate injected.
T, tumor; N, necrosis; M, muscle.
References
1. Knapp FF Jr, Beets AL, Guhlke S, Zamora PO, Bender H, Palmedo H, Biersack HJ. Availability of rhenium-188 from the alumina-based tungsten-188/rhenium-188 generator for preparation of rhenium-188-labeled radiopharmaceuticals for cancer treatment. Anticancer Res. 1997. 17:1783–1795.
2. Hsieh BT, Hsieh JF, Tsai SC, Lin WY, Huang HT, Ting G, Wang SJ. Rhenium-188-Labeled DTPA: a new radiopharmaceutical for intravascular radiation therapy. Nucl Med Biol. 1999. 26:967–972.

3. Li S, Liu J, Zhang H, Tian M, Wang J, Zheng X. Rhenium-188 HEDP to treat painful bone metastases. Clin Nucl Med. 2001. 26:919–922.

4. Buchmann I, Bunjes D, Kotzerke J, Martin H, Glatting G, Seitz U, Rattat D, Buck A, Dohner H, Reske SN. Myeloablative radioimmunotherapy with Re-188-anti-CD66-antibody for conditioning of highrisk leukemia patients prior to stem cell transplantation: biodistribution, biokinetics and immediate toxicities. Cancer Biother Radiopharm. 2002. 17:151–163.

5. Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995. 92:7297–7301.

6. Kobayashi S, Hiroishi K, Tokunoh M, Saegusa T. Chelating properties of linear and branched poly (ethylenimines). Macromolecules. 1987. 20:1496–1500.
7. Rivas BL, Geckeler KE. Synthesis and metal complexation of poly (ethylenimine) and derivatives. Adv Polym Sci. 1992. 102:173–183.
8. Zanta MA, Boussif O, Adib A, Behr JP. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug Chem. 1997. 8:839–844.
9. Sagara K, Kim SW. A new synthesis of galactose-poly (ethylene glycol)-polyethylenimine for gene delivery to hepatocytes. J Control Release. 2002. 79:271–281.
10. Kunath K, von Harpe A, Fischer D, Kissel T. Galactose-PEI-DNA complex for targeted gene delivery: degree of substitution affects complex size and transfection efficiency. J Control Release. 2003. 88:159–172.
11. Ogris M, Steinlein P, Carotta S, Brunner S, Wagner E. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS PharmSci. 2001. 3:E21.

12. Hildebrandt IJ, Iyer M, Wagner E, Gambhir SS. Optical imaging of transferrin targeted PEI/DNA complexes in living subjects. Gene Ther. 2003. 10:758–764.

13. Ogris M, Wagner E. Tumor-targeted gene transfer with DNA polyplexes. Somat Cell Mol Genet. 2002. 27:85–95.
14. Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002. 54:561–587.

15. Xu L, Pirollo KF, Chang EH. Transferrin-liposome-mediated p53 sensitization of squamous cell carcinoma of the head and neck to radiation in vitro. Hum Gene Ther. 1997. 8:467–475.

16. Kircheis R, Kichler A, Wallner G, Kursa M, Ogris M, Felzmann T, Buchberger M, Wagner E. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997. 4:409–418.

17. Stabin MG. MIRDOSE: personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 1996. 37:538–546.
18. Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999. 6:595–605.

19. Oupicky D, Konak C, Dash PR, Seymour LW, Ulbrich K. Effect of albumin and polyanion on the structure of DNA complexes with polycation containing hydrophilic nonionic block. Bioconjug Chem. 1999. 10:764–772.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download