Abstract
Acute neurologic deterioration is not a rare event in the surgical decompression for thoracic spinal stenosis. We report a case of transient paraparesis after decompressive laminectomy in a 50-yr-old male patient with multi-level thoracic ossification of the ligamentum flavum and cervical ossification of the posterior longitudinal ligament. Decompressive laminectomy from T9 to T11 was performed without gross neurological improvement. Two weeks after the first operation, laminoplasty from C4 to C6 and additional decompressive laminectomies of T3, T4, T6, and T8 were performed. Paraparesis developed 3 hr after the second operation, which recovered spontaneously 5 hr thereafter. CT and MRI were immediately performed, but there were no corresponding lesions. Vascular compromise of the borderlines of the arterial supply by microthrombi might be responsible for the paraparesis.
Thoracic spinal stenosis may result from degenerative spondylosis, ossification of posterior longitudinal ligament (OPLL), facet hypertrophy, or ossification of the ligamentum flavum (OLF) (1). Chronic severe myelopathy caused by thoracic spinal stenosis can be reversible with appropriate decompression. Neurological deterioration after surgery is a serious complication. Prevention or immediate recovery is important, however, not always possible. Paraplegia during medical treatment may result from either procedures close to the spinal cord, such as laminectomy, vertebrotomy, spondylodesis, and peridural anesthesia, involving the risk of mechanical damage to the spinal cord, or procedures distant from the spinal cord, such as vascular surgery, angiography, radiotherapy, bronchial artery remobilization, and umbilical artery injection (2). Disturbances of the blood supply or toxic mechanisms may be responsible, too. Transient paraplegia after a decompressive laminectomy for the thoracic stenosis is unusual. This article describes such a case, including clinical presentation, management, and reviews the risk of neurological deterioration.
A 50-yr-old man experienced transient paraparesis for 3 to 5 min after an accidental extension of his back in March 2000. His legs turned back soon and became normal. He presented himself to the authors one month later. He felt back pain and paresthesia on both lower extremities. The paresthesia was more severe on the left side. Physical examination showed mild ankle clonus on both sides. Knee jerks were hyperactive. Babinski response was normal on both sides. Straight leg raising test was normal on both sides. An electromyography revealed lower lumbar radiculopathy. However, magnetic resonance imaging (MRI) of the lumbar spine revealed no pathological findings.
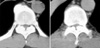
In October 2002, he visited again due to severe tingling sensation disturbing his sleep. Physical examination showed similar features. MRI of the thoracic spine revealed OLF at the T9, T10, and T11 (Fig. 1). Decompressive laminectomy from T9 to T11 was performed. Despite surgery, his gross neurological status was not improved. Post-operative computed tomographic scan (CT) of the thoracic and cervical spine revealed additional lesions (Fig. 2); OPLL of the cervical spine (from C4 to C6) and OLF of the upper thoracic spine (from T3, 4, T6, and T8). Two weeks after the initial operation, additional operation was planned. Laminoplasty for the lower cervical spine (C4 to C6) and additional decompressive laminectomy of the thoracic spine (T3, T4, T6, and T8) were performed. On operation, there was epidural bleeding at C4, which was controlled by a gelfoam. There were no episodes of hypoxia or hypotension. In the recovery room, he could move all his extremities. He was moved back to his ward and was stable. Three hours later, however, he became paraplegic, without any changes in his vital signs. He could not move his toes and he was anesthetic below the level of the nipple. CT and MRI were immediately performed, but there were no corresponding lesions. As a possible cause of the deterioration, we suggested distortion of the cord at the compression-decompression junction. So, emergency operation for a complete laminectomy to resolve the cord kinking was planned. However, the patient and his relatives refused the emergency operation. Surprisingly, he could move his legs spontaneously, about five hours later (eight hours after the second operation). His motor functions were improved to grade 3. Four days later, we drilled out all the laminae of the thoracic spine from T2 to T11 to prevent a possible cord kinking. Although there was mild hypesthesia, his motor function improved subsequently. He was discharged walking without any aids on the 34th hospital day. Although his symptoms were stable for several months, they started to deteriorate slowly. Four months after the last operation, his symptoms were almost the same as the pre-operative status. Now, he is on an alternative medicine without additional improvements.
Thoracic stenosis from OLF is not uncommon in Korea (3-8). Surgical treatment, particularly laminectomy, was usually successful and the outcomes were very promising. However, there is a risk of acute neurologic deterioration. The incidence of paraplegia through medical treatment was reported at 0.69% in an orthopedic university hospital (2). For thoracic spinal stenosis, acute neurologic deterioration is quite common after a posterior decompressive laminectomy. Young and Baron reported the incidence as high as 14.5% through a review of the literature (9). Although there have been a few cases of transient paraparesis after surgery (10-12), it is rare that transient paraparesis should occur after decompressive laminectomy and subsequently improve. Transient or temporary paraparesis may result from intraspinal hematomas (13, 14), acute aortic dissection (15), general anesthesia (16), or epidural anesthesia (17).
Direct trauma during operation or postoperative hematomas might be a cause of paraparesis. However, the patient could move all his extremities after the surgery. In addition we could not find any postoperative hematomas or any compressive lesions as in other authors' experiences (9). Distortion of the spinal cord may occur at the compression-decompression junction (1). The altered cerebrospinal fluid flow dynamics may cause cord compression (11). However, spontaneous recovery before thorough decompressive laminectomy does not support such an assumption. There are several theories for neurologic deterioration after decompressive laminectomies, such as vascular compromise, hypotension or ischemia, direct trauma, or stretching of the neural elements. Some proposed the long duration of symptoms, multiple sites of compression (1), or the degree of preoperative thoracic stenosis in CT scan (18) as a bad prognostic factor. The surgical outcome is poor in patients whose initial symptoms had lasted for more than 2 yr, and who had additional proximal stenosis. The outcome is poor especially when the distal decompression was carried out first (1). We performed the distal decompression first, however, transient paraparesis occurred after the second proximal decompression. We do not know the exact mechanism of transient paraparesis of this patient. Anesthesia below the level of the nipple (T4) and spontaneous recovery suggested that vascular compromise of the borderlines of the arterial supply by microthrombi might be responsible for the paraparesis.
Decompressive laminectomy was usually successful for the OLF. However, the rate of complication was reported as high as 13.9% (19). The risk of acute neurologic deterioration is not low in the surgical decompression for the thoracic spinal stenosis. Surgeons should be alert to such complications. It is necessary to explain the rate of neurologic deterioration and possible complications after surgery before operative intervention, especially in those patients with symptoms of long duration, severe myelopathy, or multi-level involvement.
Figures and Tables
References
1. Chang UK, Choe WJ, Chung CK, Kim HJ. Surgical treatment for thoracic spinal stenosis. Spinal Cord. 2001. 39:362–369.

2. Bacher T, Schiltenwolf M, Niethard FU, Paeslack V. The risk of paraplegia through medical treatment. Spinal Cord. 1999. 37:172–182.

3. Cho YH, Moon SM, Roh SW, Joen SR, Rhim SC. Surgical outcome and prognostic factors of ossified ligamentum flavum of the thoracic spine. J Korean Neurosurg Soc. 2002. 32:424–430.
4. Kim J, Cho JH, Oh HK. Thoracic myelopathy due to ossification of ligamentum flavum: three cases report. J Korean Soc Spine Surg. 2002. 9:257–261.
5. Kim YS, Jin BH, Yoon DH, Cho YE, Chin DK. Thoracic stenosis secondary to ossification of the ligamentum flavum. J Korean Neurosurg Soc. 1997. 26:971–979.
6. Park YS, Lee IH, Kim MK. A case of myelopathy due to the nodular ossification of the ligamentum flavum of thoracic spine in diffuse idiopathic skeletal hyperostosis. J Korean Rheum Assoc. 2000. 7:174–178.
7. Vasudevan A, Knuckey NW. Ossification of the ligamentum flavum. J Clin Neurosci. 2002. 9:311–313.

8. Yoo MC, Kim KT, Kim YW, Kim HS, Jeon MH. Sacral radiculopathy due to ossification of ligamentum flavum and posterior longitudinal ligament: one case report. J Korean Orthop Assoc. 1998. 33:834–839.

9. Young WF, Baron E. Acute neurologic deterioration after surgical treatment for thoracic spinal stenosis. J Clin Neurosci. 2001. 8:129–132.

10. Li KK, Chung OM, Chang YP, So YC. Myelopathy caused by ossification of ligamentum flavum. Spine. 2002. 27:E308–E312.

11. Tribus CB. Transient paraparesis: a complication of the surgical management of Scheuermann's kyphosis secondary to thoracic stenosis. Spine. 2001. 26:1086–1089.
12. Valls PL, Naul LG, Kanter SL. Paraplegia after a routine lumbar laminectomy: report of a rare complication and successful management. Neurosurgery. 1990. 27:638–640.

13. Hentschel SJ, Woolfenden AR, Fairholm DJ. Resolution of spontaneous spinal epidural hematoma without surgery: report of two cases. Spine. 2001. 26:E525–E527.
14. Gerancher JC, Waterer R, Middleton J. Transient paraparesis after postdural puncture spinal hematoma in a patient receiving ketorolac. Anesthesiology. 1997. 86:490–494.

15. Tanaka T, Uemura K, Sugiura M, Ohishi H, Tomita M, Nagasaki F, Matsuda S, Kobayashi R, Fukaya T. Transient paraplegia caused by acute aortic dissection. Neurol Med Chir (Tokyo). 1990. 30:54–58.

16. Kim C, Blank J, McClain BC. Transient paraparesis after general anesthesia in a patient in the prone position. Anesthesiology. 1994. 81:775–777.

17. Chiapparini L, Sghirlanzoni A, Pareyson D, Savoiardo M. Imaging and outcome in severe complications of lumbar epidural anesthesia: report of 16 cases. Neuroradiology. 2000. 42:564–571.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download