Abstract
An animal model of spinal cord trauma is essential for understanding the injury mechanisms, cord regeneration, and to aid the development of new therapeutic modalities. This study focused on the development of a graded experimental contusion model for spinal cord injury (SCI) using a pneumatic impact device made in Korea. A contusive injury was made to the dorsal aspect of the cord. Three trauma groups were defined according to the impact velocity (IV). A control group (n=6), received laminectomy only. Group 1 (n=10), 2 (n=10), and 3 (n=10) had IVs of 1.5 m/sec, 2.0 m/sec, and 3.5 m/sec respectively. Functional assessments were made up to the 14th day after injury. The cord was removed at the 14th post-injury day and prepared for histopathologic examination. Significant behavioral and histopathological abnormalities were found in control and each trauma group. All trauma groups showed severe functional impairment immediately after injury but following different rates of functional recovery (Fig. 5). As the impact velocity and impulse increased, the depth of contusive lesion revealed to be profound the results show that the rat model reproduces spinal cord lesions consistently, has a distinctive value in assessing the effects of impact energy.
Several investigators have developed animal models of spinal cord injury (SCI) in an attempt to reproduce various aspects of biomechanical responses, neurological deficits, and pathology. The first well-controlled animal model of spinal cord injury by using a weight-drop technique was described by Allen in 1911 (1). The most common method for production of SCI is the weight-drop method (2). In recent years, artificial means of acceleration have been used to produce spinal cord injury with devices that are capable of generating and recording a wide range of impact parameters (1).
The latest device to make an experimental model of SCI is the pneumatic impact device. The pneumatic impact device was first applied in the controlled cortical impact model of traumatic brain injury (3-6). The advantage of a pneumatic impact device is that the rate and amount of tissue displacement can be controlled independently of the properties of the spinal cord as a mechanical system (1). There are some domestic papers about an experimental model of SCI by using weight drop method or cord compression. However, there is no paper by using a pneumatic impact device so we made the device and develop an rat model of SCI.
The aims of this study are to develop a reliable and reproducible graded contusive SCI model in rats with the pneumatic impact device made in authors' institution as well as to estimate performance of the device, and to investigate the effect of the degree of impact energy on the functional outcome and histopathologic findings of the SCI model.
The Clinical and Experimental Studies Committee of Chung-Ang University School of Medicine approved this study. A total of 36 male Sprague-Dawley rats average weighing 228±24 g were used. Animals were 7 to 9 weeks old and were randomly distributed to the experimental and control groups. Food and water were kept freely available during the perioperative period.
The rats were anesthetized initially with ketamine hydrochloride and xylazine hydrochloride mixed solution (Ketamine: Xylazine=3:1, i.m.), and maintained by inhalation of 2% halothane mixed with oxygen and compressed air.
After a deep level of anesthesia was obtained, the rat was placed in a prone position on the table and a midline dorsal incision was made in the skin from T8 to T13 levels. Muscles around T9 to T11 were incised and retracted on both sides of the vertebral column to expose the spinous processes. Total laminectomy of T9 or T10 was done with preservation of the dura. Because the impact tip diameter was 2.5 mm, the exposed surface of the spinal cord had to be larger than 3 mm in diameter.
Then, the animal was placed in a prone position at the pneumatic impact device and its head was fixed with the Kopf stereotactic frame (David Kopf Instruments, Tujunga, CA, U.S.A.) using the ear bar, incisor bar, and head holder. The spinal column was not suspended to lessen surgical insults and for simplicity. Six rats received T9 or T10 laminectomy without an impact on the spinal cord and were included in the control group.
The key settings for the impact were 0.2 sec dwell time, 2 mm depth of deformation, and 1/3,000 sec scan rate. The target point was the midline dorsal aspect of the spinal cord. The animals were divided into 3 experimental groups according to impact velocity (IV). In group 1, the IV was 1.5 m/sec at a force of 40 pounds per square inch (PSI) with a closed quick valve (n=10). In group 2, the IV was 2.0 m/sec at 35 PSI with an open quick valve (n=10). In group 3, the IV was 3.5 m/sec at 100 PSI with an open quick valve (n=10). After the impact, the wound was closed in layers using aseptic technique.
Lighthall introduced a new experimental brain injury model that could control the cortical impact using a stroke-constrained pneumatic impactor in 1988 (4). We developed a new controlled cortical impact device, using the type of pneumatic impact device that had been described in detail by Lighthall, with technical advice from the Department of Mechanical Engineering at the Korea Advanced Institute of Science and Technology (KAIST) (1, 3-7). We completed the "Chung-Ang University Hospital Model 1.0 (CAUH-1)" based on a controlled cortical impact model in August, 2001. The device was applied to a traumatic brain injury model in rats at first (8, 9). We upgraded CAUH-1 by adding a sensor and replacing the previous load cell which detect the amount of impulse and developed the CAUH-2 (Fig. 1). Recently, the controlled cortical impact technique has been improved in which the biomechanical events contributing to injury can be analyzed by establishing a quantifiable relationship with measurable engineering parameters such as force, velocity, depth of compression and dwell time (1, 3, 7).
The pneumatic impactor consists of a small bore, double-acting stroke-constrained pneumatic cylinder with a 5.0 cm stroke. The cylinder is rigidly mounted in a vertical position on a crossbar, which can be precisely adjusted in the vertical axis. The lower end of the rod has a changeable impact tip. The upper end of the rod is attached to the transducer core of a linear variable differential transformer (LVDT). Impact velocity is calculated from the time/displacement curve that is measured by the LVDT (AMSYS, Korea). The sensors for force and acceleration are located on the impactor tip. Output from the LVDT, force sensor and acceleration sensor are stored and analyzed on a PC-based data acquisition system (Labview program, National Instrument, Austin, Texas, U.S.A.). The impact velocity can be adjusted between 1.25 and 3.55 m/sec by controlling gas pressure. Fig. 2 shows the relationship between the driving pressure applied to the impactor and the velocity of the impact tip.
Fourteen days after the injury, the rats were reanesthetized and the spinal cords including the experimental lesions were dissected out as one piece, and fixed with 4% buffered paraformaldehyde solution for 24 hr. Spinal cords were then embedded in paraffin, cut into 5 µm-thick axial and sagittal plane slices, and stained with hematoxylin and eosin (H&E), staining with Luxol fast blue (LFB) to identify myelinated white matter and residual tissue sparing was also done.
All rats were examined in the open field environment to test locomotion functions of their hind limbs prior to injury, and at 0, 1, 3, 5, 7, 10 and 14th post-injury days using the Basso, Beatie and Bresnahan (BBB) Locomotor Rating Scale, hind foot bar grab, and platform hang (2, 10-12). All tests were examined by two observers. When the hind limb score of one side was different from the other, the worse score was used for data.
Motor functions of hind limbs were assessed by the BBB score, for example, the rate would be 0 (the lowest rate) if the rat showed no hindlimb motion or 21 (the highest rate) if the rat showed normal motion (10, 11). The hind foot bar grab test was useful to test the function of polysynaptic spinal reflexes, for example, the score would be 0 (the lowest score) if the rat did not respond to touch of bar on hind feet or 3 (the highest score) if the rat strongly grabbed the bar and thrust it (2). The platform hang test was useful to test the coordinated motor function of the hind limbs, for example, the score would be 0 (the lowest score) if the rat fell onto soft bed or 4 (the highest score) if the rat climbed the platform within 5 sec (12). The data of the various behavioral tests are provided as mean values (mean±standard deviation).
The functional outcomes were compared between groups using one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparison post-hoc test at each post-injury time point. We analyzed the relationship between the impact velocity and impulse by t-test and Mann-Whitney Rank Sum Test. p values of <0.05 were considered statistically significant.
During the procedures blood loss was minimal but three rats died as a result of procedures. Only one case of wound infection occurred with a superficial purulent abscess, which was remedied by a single wound incision. No animal received antibiotics. Thirty-three of the 36 rats survived the full 14 days of the study. All the surviving rats receiving the cord trauma presented definite lesions at the contused cord area.
Contusive injuries of increasing severity produced concomitant progressive degradations in behavioral performance (Fig. 5). The control group showed no functional deficit. Generally, all the animals with cord injury exhibited profound functional impairment immediately after the trauma, with varying degrees of functional recovery over 2 weeks.
Group 1 exhibited a rapid functional recovery during the first and second days after trauma in hind foot bar grip and platform hang test. The BBB score increased slowly during the seven days. Group 2 was similar with Group 1 in the course of functional recovery, although it showed moderate functional deficits in the BBB score (Fig. 5). However, there was little improvement in the hind foot bar grip as time passed. There was no significant difference between group 2 and 3 in platform hang test (Table 1). Group 3 presented severe deficits in all three functional scales immediately after injury, and the deficits continued until the 14th day post-injury. Only the hind foot bar grab score showed some improvement after the 7th day of injury.
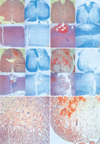
A histological examination of the contused spinal cord showed hemorrhage and necrosis mainly involving the midline dorsal portion of the spinal cord and tapering as it went to the deep portion. As the impact velocity and impulse increased, the depth of the lesions seemed to be greater (Fig. 6). The white matter area stained with LFB represents myelinated fibers that survived injury. In Group 1, the lesion occupies in the dorsal column with minimal tissue destruction and shows hemorrhage in the white matter. There was extensive white matter sparing, which was proven by a peripheral rim of blue stain. The gray and white matter in the ventral half of the cord was normal (Fig. 6B). In Group 2, the lesion involved the gray and white matter in the dorsal half of the cord. The area of intact white matter was limited to a portion of the lateral and ventral funiculus (Fig. 6C). Microcystic changes in the area of intact white matter are observed (Fig. 6E). In Group 3, the lesion occupied almost the entire cross-sectional area of the cord, with a narrow area of spared white matter was at the ventral side of the cord (Fig. 6D). The number of microcysts around the area of intact white matter was greater in Group 3 than the other groups (Fig. 6F).
The greater the severity of injury, the more frequent was microcystic formation. Increased impulse energy was responsible for the increased neuronal cell loss. Thick and dense staining with LFB in the white matter of the cord was scattered in appearance because of the microcysts within the area of diffuse axonal injury (13, 14). This phenomenon is the result of the propagation of the impact pulse to the area of intact white matter.
Experimental groups based on the amount of pneumatic impact showed significant differences in both functional and histopathological outcome measures. Recently, various methods of creating experimental SCI have been developed (2, 10, 15, 16). The pneumatic impact device has some merits compared to the traditional weight-drop model. The principle determinants of this device such as velocity, depth, and duration of compression and impulse are controllable, and measurable. These controlled mechanical variables enable the amount of deformation and the change in deformation over time to be determined accurately. And, there is no secondary injury, such as from a dropped weight rebounding on the dura after impact (2). Since the other methods do not allow independent control of these variables, the interaction of these factors has not been fully elucidated.
The BBB Scale, an open field scoring system that requires little time to perform, has been reported to assess motor functions of rats more precisely than some methods (2, 11, 12). The BBB score consistently correlated with functional results in each group, and the results clearly showed graded impairment of motor function according to the IV. However, the scores of platform hang and hind foot bar grab after the 7th post-injury day were very inconsistent between group 2 and 3. The histopathologic findings between group 2 and 3 are distinguishable. In the rat model of contusive SCI, the area of white matter at the lesion was considered the best single measurement for characterizing the degree of injury, providing a simple but accurate factor related with the degree of the spinal cord injury (17). Our SCI model using the pneumatic impact device is the first in Korea. It has the advantage that variable parameters can be measured concurrently in a single experiment.
The question of obvious interest is whether the rat model of contusive SCI is applicable to human SCI. At this point, there is not enough information to answer this question. However, we think the rat model may be useful for testing potential therapeutic interventions and presents a chance to investigate how differences in cellular responses to injury affect functional outcomes and mechanisms of recovery.
Figures and Tables
Fig. 1
Photograph and diagram of pneumatic impact device (CAUH-2). A pneumatic impact controller regulates the flow of high pressure gas to the pneumatic piston. A, Gas; B, Sensor; C, Controller; D, Amplifier; E, Data acquisition board; F, Computer.

Fig. 2
Graph showing the relationship between the driving pressure to the impactor (range, 30-110 psi) and the velocity of the impact tip.
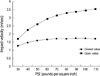
Fig. 4
Graph showing the relationship between the impact velocity (A) and impulse (B) in each group. (Value: mean±standard deviation).
*p<0.05 vs. group 1, †p<0.05 vs. group 2.

Fig. 5
The time course of functional recovery as measured for the control and experimental groups. (A) The BBB score, (B) Hind foot bar grab, (C) Platform hang.

Fig. 6
Representative coronal and sagittal sections of the lesions 14 days after the injury. The dorsal surface is at the top. The left panels are hematoxylin and eosin stain and the right are Luxol fast blue stain (Original magnification, ×40). The Luxol fast blue stain clearly reveals residual myelinated white matter that is restricted to a peripheral rim in the more severe injury groups. (A) control, (B) Group 1, (C) Group 2, (D) Group 3, (E, F) Higher power magnification of the ventral-lateral funiculus (the block demarcates the region of spared white matter, ×100). *: Cystic formation. (E, F) Higher power magnification of the ventral-lateral funiculus (the block demarcates the region of spared white matter, ×100).
References
1. Andrew RB. Povlishock JT, editor. An overview of spinal cord injury models. Neurotrauma. 1996. New York: McGraw-Hill;1367–1379.
2. Seki T, Hida K, Tada M, Koyanagi I, Iwasaki Y. Graded contusion model of the mouse spinal cord using a pneumatic impact device. Neurosurgery. 2002. 50:1075–1081.

3. Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991. 39:253–262.
4. Lighthall JW. Controlled cortical impact: A new experimental brain injury model. J Neurotrauma. 1988. 5:1–15.

5. Lighthall JW, Dixon CE, Anderson TE. Experimental models of brain injury. J Neurotrauma. 1989. 6:83–97.

6. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994. 80:291–300.
7. Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987. 67:110–119.

8. Choi SM, Suk JS, Kwon JT, Min BK, Kim YB, Hwang SN, Choi DY, Kim JH, Lee SM, Earmme YY. Development of upgraded cortical impact model (Part I: Mechanics). J Korean Neurosurg Soc. 2002. 32:29–34.
9. Choi SM, Suk JS, Min BK, Hwang SN, Kim YB, Kim JH. Development of upgraded cortical impact model (Part II: Functional outcome). J Korean Neurosurg Soc. 2002. 32:458–462.
10. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outocmes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996. 139:244–256.
11. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–12.

12. Kuhn PL, Wrathall JR. A mouse model of graded contusive spinal cord injury. J Neurotrauma. 1998. 15:125–140.

13. Bresnahan JC. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J Neurol Sci. 1978. 37:59–82.

14. Bresnahan JC, King JS, Martin GF, Yashon D. A neuroanatomical analysis of spinal cord injury in the rhesus monkey (Macaca mulatta). J Neurol Sci. 1976. 28:521–542.

15. Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, Stokes BT. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000. 17:299–319.

16. Ma M, Basso DM, Walters P, Strokes BT, Jakeman LB. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57B1/6 mouse. Exp Neurol. 2001. 169:239–254.
17. Noble LJ, Wrathall JR. Spinal cord contusion in the rat: Morphometric analyses of alteration in the spinal cord. Exp Neurol. 1985. 88:135–149.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download