Abstract
Persistent truncus arteriosus categories associated with different natural histories and various surgical approaches were reported. Although pulmonary overflow and severe heart failure are common, some patients who have hypoplastic pulmonary artery systems may show lesser symptoms of heart failure and remain in relatively stable condition. We experienced a 33-year-old woman with uncorrected type II persistent truncus arteriosus who presented with cyanosis rather than congestive heart failure, and are presenting her images.
Persistent truncus arteriosus (PTA), a rare congenital cardiac malformation, comprises approximately 1% of congenital heart defects. Because of pulmonary overflow and severe heart failure (HF), older patients who have not undergone repair have the risk of developing severe vascular occlusive disease of the pulmonary tree and early death.1) However, some patients who have hypoplastic pulmonary artery systems may show lesser symptoms of HF and remain in relatively stable condition.2)3)4) We experienced a 33-year-old woman with uncorrected type II PTA who presented with cyanosis rather than congestive HF, and herein present her case.
A 33-year-old woman with cyanosis, dizziness, and dyspnea at rest came to the adult cardiology clinic. Her history revealed that she had been diagnosed with ventricular septal defect (VSD) at the age of 1 month. The patient was in functional class III during the preceding year with a good response to low-dose diuretic treatment.
On physical examination, her height and weight were 140 cm and 40.9 kg. She showed central cyanosis and clubbed fingers. There was no heart murmur except P2 accentuation. No signs of definite congestive heart failure (CHF) were found. Her blood pressure was 100/70 mm Hg, heart rate was 85 beats/min, and respiratory rate was 22 breaths/min. Arterial blood oxygen saturation without oxygen was 78%. Blood count showed a hemoglobin level of 12.5 mg/dL and hematocrit of 41.7%. Renal and liver function tests were normal. Her N-terminal pro-B type natriuretic peptide level was 432.4 pg/mL and cardio-high sensitivity C-reactive protein was 0.01 mg/dL.
A chest radiograph showed increased pulmonary vascularity with cardiomegaly (cardiothoracic ratio = 0.61). Electrocardiogram (ECG) showed normal sinus rhythm, right atrial enlargement, and mild biventricular hypertrophy. On the transthoracic echocardiographic examination, parasternal long and short axis view showed overriding a non-restrictive VSD supplied by both ventricles, a single great vessel overriding both ventricles from the base of the heart, absence of the main pulmonary arterial trunk, and adjacent right and left pulmonary arteries (PAs) that arose separately from the dorsal aspect of the common trunk (Fig. 1). The size of the origins of the right and left PAs in bifurcation from the main PA were 12 mm (Z-score = -1.3) and 8 mm (Z-score = -5.7), respectively. The annulus size of common truncus was 30 mm. An apical 4 chamber view revealed a hypertrophied right ventricle, normal function of the left ventricle (LV), and mild tricuspid regurgitation (TR). The systolic flow velocity of TR was 4.9 m/s and interventricular septum during the systolic phase was flat, systolic D shaped LV (Fig. 2). ECG-gated multidetector computed tomography was performed, revealing the same features as echocardiography (Fig. 3 and 4). Since the imaging studies confirmed that two PAs arose from the posterior aspect of the truncus separately but close to each other, PTA type II was diagnosed. Although we planned cardiac catheterization and bosentan therapy, she refused further evaluation and medication. Her condition was relatively stable and a conservative management regimen (low-dose diuretic treatment) was maintained.
The natural history of PTA is poor. The mortality rate is as high as 80% within the first year of life without surgical correction.1) A few unrepaired patients with long survival were described in previous case reports.2)3)4) Among these cases, one with type IV PTA is remarkable, showing greater longevity than the others.2) Although this patient did not have well-developed PAs, sufficient circulation to the PAs by means of collateral vessels and balanced systemic circulation may have been present. This probably resulted from protection of the pulmonary circulation induced by the brachial artery or hypoplastic branch PAs in this type IV PTA patient.
Neonates have high perinatal pulmonary vascular resistance (PVR) that gradually decreases after birth. The amount of pulmonary blood flow is dependent on the degree of PVR. Although PVR is decreased, PA hypoplasia in patients with PTA may induce protection of the pulmonary circulation and thus may be beneficial. A previous study on six adults with PTA reported that long survival is related to hypoplasia of PA branches.5) The authors mentioned that PA hypoplasia or stenosis was associated with decrease of pulmonary blood flow and protection against early CHF.
Recently, PTA categories associated with different natural histories and various surgical approaches were reported.6) Dominance was defined as pulmonary in patients with hypoplastic ascending aorta and either aortic interruption or severe coarctation; as aortic in patients with brachiocephalic arteries supplied by a common trunk and PAs originating from its dorsal aspect. Survival rates in postoperative prognosis were 84% in those with pulmonary dominance and 66% in those with aortic dominance. Although the paper did not comment on symptoms related to CHF, it may be suspected that patients with pulmonary-dominant PTA may exhibit intractable CHF due to PTA in the neonatal period. Our patient also had hypoplasia in the origin of the PA that can be classified as aortic-dominant PTA. Therefore, in spite of cyanosis, her condition was relatively stable, as described in previous cases.
Patients with PTA are at risk for pulmonary vascular occlusive disease.7)8) They need management for right ventricular failure and medication with pulmonary vasodilators.
The present case indicates that the natural course of PTA is associated with particular PA anatomy and morphology.
Figures and Tables
Fig. 1
The images of parasternal long and short axis view of transthoracic echocardiography. A parasternal long axis view showed an overriding non-restrictive ventricular septal defect (VSD) supplied by both ventricles (A), and a single great vessel overriding both ventricles from the base of the heart (B). A parasternal short axis view showed absence of the main pulmonary arterial trunk, and adjacent right and left pulmonary arteries (PAs) that arose separately from the dorsal aspect of the common trunk (C). The size of the origins of the right and left PAs in bifurcation from the main PA were 12 mm (Z-score = -1.3) and 8 mm (Z-score = -5.7), respectively. RV: right ventricle, LV: left ventricle, LA: left atrium, RPA: right pulmonary artery, LPA: left pulmonary artery.

Fig. 2
An apical 4 chamber view revealed a hypertrophied right ventricle (A). The systolic flow velocity of TR was 4.9 m/s (B) and interventricular septum during the systolic phase was flat, systolic D shaped LV (C). RV: right ventricle, LV: left ventricle.

Fig. 3
Electrocardiogram-gated multidetector computed tomography showed pulmonary arteries arising separately from the dorsal aspect of the common trunk (A and B), overriding non-restrictive ventricular septal defect supplied by both ventricles (C), hypertrophied right ventricle and flat interventricular septum (D).
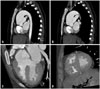
Fig. 4
Two images acquired from three dimensional reconstruction of electrocardiogram-gated multidetector computed tomography showed that two pulmonary arteries arose from the posterior aspect of the truncus separately but close to each other. Antero-lateral aspect (A) and dorsal aspect (B). RPA: right pulmonary artery, LPA: left pulmonary artery.

References
1. Slavik Z, Keeton BR, Salmon AP, Sutherland GR, Fong LV, Monro JL. Persistent truncus arteriosus operated during infancy: long-term follow-up. Pediatr Cardiol. 1994; 15:112–115.
2. Bodí V, Insa L, Sanchis J, Ibáñez M, Losada A, Chorro FJ. Persistent truncus arteriosus type 4 with survival to the age of 54 years. Int J Cardiol. 2002; 82:75–77.
3. Hicken P, Evans D, Heath D. Persistent truncus arteriosus with survival to the age of 38 years. Br Heart J. 1966; 28:284–286.
4. Guenther F, Frydrychowicz A, Bode C, Geibel A. Cardiovascular flashlight. Persistent truncus arteriosus: a rare finding in adults. Eur Heart J. 2009; 30:1154.
5. Espínola-Zavaleta N, Muñoz-Castellanos L, González-Flores R, Kuri-Nivón M. [Common truncus arteriosus in adults]. Arch Cardiol Mex. 2008; 78:210–216.
6. Russell HM, Jacobs ML, Anderson RH, Mavroudis C, Spicer D, Corcrain E, Backer CL. A simplified categorization for common arterial trunk. J Thorac Cardiovasc Surg. 2011; 141:645–653.
7. Marcelletti C, McGoon DC, Mair DD. The natural history of truncus arteriosus. Circulation. 1976; 54:108–111.
8. Boris JR. Primary care management of patients with common arterial trunk and transposition of the great arteries. Cardiol Young. 2012; 22:761–767.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download