Abstract
Background and Objectives
Intravenous immunoglobulin-SN (IVIG-SN) is a new human immunoglobulin product. Its safety is ensured by pathogen-elimination steps comprising solvent/detergent treatment and a nanofiltration process. This multicenter clinical study was designed to evaluate the efficacy and safety of combined aspirin and high-dose IVIG-SN therapy in pediatric patients with Kawasaki disease (KD).
Subjects and Methods
We evaluated coronary artery lesions (CALs) at 2 and 7 weeks after administering IVIG-SN; total fever duration; and variations in erythrocyte sedimentation rate, N-terminal pro B-type natriuretic peptide or B-type natriuretic peptide, and creatine kinase-myocardial band level before and after treatment with IVIG-SN (2 g/kg). Adverse events were monitored.
Results
Forty-five patients were enrolled, three of whom were excluded according to the exclusion criteria; the other 42 completed the study. The male:female ratio was 0.91:1, and the mean age was 29.11±17.23 months. The mean fever duration before IVIG-SN treatment was 6.45±1.30 days. Although most patients had complete KD (40 patients, 90.91%), four had atypical KD (9.09%). After IVIG-SN treatment, one patient (2.38%) had CALs, which was significantly lower than the incidence reported previously (15%) (p=0.022), but not significantly different from recent data (5%). There were no serious adverse events, though 28 patients (63.64%) had mild adverse events. Three adverse drug reactions occurred in 2 patients (eczema, anemia, and increased eosinophil count), all of which were transient.
Kawasaki disease (KD) is an acute, febrile vasculitis of unknown cause that is most frequently observed in children under 5 years of age. It usually invades medium-sized blood vessels and, if left untreated, leads to complications in the coronary arteries in approximately 20-25% of cases,1)2)3) possibly causing myocardial infarction or sudden death.4) The disease has spread worldwide since it was first reported in Japan in 1967, and its incidence is significantly higher in the Asian population than in the Western population.5)6)7) A nationwide survey performed in Korea revealed that over 4000 patients had KD in 2011, which is the second highest incidence of KD worldwide, after Japan.8)
The current widespread treatment of patients with acute KD includes a single, high-dose administration of intravenous immunoglobulin (IVIG) (2 g/kg/day, single-dose therapy), combined with oral, high-dose aspirin (80-100 mg/kg/day, divided-dose therapy). This regimen can reduce the incidence of coronary artery complications from 15-25% to 5% if administered within 10 days of the onset of fever.9)10)11) Although the causes and pathogenesis of KD remain unknown, the clinical effects of IVIG on KD have been known for a long time. To date, neutralization of the unidentified antigens or toxins that cause KD, inhibition of immune complexes that act on vascular cells through the fragment crystallizable-portion of immunoglobulin G (IgG), or negative feedback caused by a high IgG concentration have all been hypothesized to inhibit antibody production, reduce the amount of soluble immune complexes, and inhibit platelet adhesion and thrombokinesis in vessel walls.12) Current clinical results regarding the relationship between IVIG and the incidence of coronary arterial lesions (CALs) caused by KD are not consistent.13)14)15)
IVIG-SN™ (IV-Globulin SN™, Green Cross Corporation, Yongin, Korea) is a new globulin agent with improved safety compared with the previous IVIG-S™ (IV-Globulin S™, Green Cross Corporation, Yongin, Korea) due to three extra steps in the manufacturing process (in addition to cold ethanol fractionation and ion-exchange chromatography) to eliminate viruses: fraction III precipitation, solvent/detergent (S/D) treatment, and nanofiltration processes. IVIG-S has been used for over 10 years in Korea and other countries, such as Brazil, India, and Iran. IVIG-SN has the same formula as IVIG-S with improved product safety and quality. The present clinical trial sought to assess the efficacy and safety of IVIG-SN in patients with KD.
The IVIG-SN™ (IV-Globulin SN™) used in this study was manufactured from human plasma that was collected and tested by a donor center that is licensed by the Ministry of Food and Drug Safety of Korea. Each donation was tested for parvovirus B19, hepatitis B and C viruses (HCV), and human immunodeficiency viruses 1 and 2 (HIV 1/2). Plasma pools were also tested for hepatitis B surface antigen, HIV, and HCV by enzyme immunoassay and for HCV, HIV, and B19 by nucleic acid amplification technology. The manufacturing process for this product included Cohn-Oncley cold ethanol fractionation, ion-exchange chromatography, and three additional steps dedicated to eliminating viruses: fraction III precipitation, virus inactivation by S/D treatment, and nanofiltration. Formulated as a solution, IVIG-SN™ contains 45-55 mg/mL of protein (96% IgG) and 100 mg/mL of maltose and has a pH of 4.25.
Children with KD (complete or incomplete) aged 3 months to 7 years were enrolled in this study. Pediatric patients with complete KD were eligible even if they were older than 7 years. Atypical KD patients were also included. Complete KD was defined as four or more of the major symptoms accompanied by fever lasting >5 days.4) Atypical KD was defined as three or more symptoms accompanied by fever lasting >5 days. CALs were detected by echocardiography in this study.
The exclusion criteria were the administration of other clinical products within 30 days before the onset of this clinical trial; a platelet count <100000/mm3; a white blood cell (WBC) count <3000 cells/mm3; a hemoglobin, hematocrit, or red blood cell count more than 30% above the upper limit or 30% below the lower limit of the normal range; and administration of an immunosuppressant or immune-modifying drug within 3 months before treatment. All patients' parents or legal guardians provided written informed consent, and this study was approved by the institutional review boards and ethics committees of all participating institutions.
This clinical trial was designed as a multicenter, single-arm, open-label clinical trial. The patients were administered a single dose of IVIG-SN for at least 12 h based on the usage and dosage described in the protocol (2 g/kg/day, single-dose therapy), in combination with aspirin as a concomitant medication. Each patient's parent or legal guardian measured the body temperature (at the ear drum) every 4 h after administering IVIG-SN, until the body temperature fell below 37.5℃ and then every 8 h until hospital discharge. The body temperatures were recorded in a daily log book. The same dose was repeated at the investigator's discretion if a normal body temperature (37.5℃ or below) was not maintained and none of the clinical symptoms had improved at 36 h after treatment. The patient was disqualified if normal body temperature was not maintained and none of the clinical symptoms improved within 36 h after the second IVIG-SN treatment. The efficacy and safety were assessed at screening, IVIG infusion, admission, 2 weeks after IVIG treatment (day 14±4), and 7 weeks after IVIG treatment (day 49±7). Aspirin was administered as a concomitant medication orally at 50 mg/kg/day in three divided doses until week 2 and at 5 mg/kg/day in a single dose from week 2 until the final visit. The efficacy and safety of IVIG-SN were evaluated during the visits at week 2 and week 7.
This study was conducted in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. This trial has been registered at ClinicalTrials.gov as NCT01524939.
CALs were the primary efficacy measure in this study and were defined in accordance with the CAL criteria announced by Japan's Ministry of Health, Labor, and Welfare. This clinical trial used echocardiography to diagnose CALs, which is more convenient and less invasive than other diagnostic methods and is widely used in clinical settings. CALs were defined as an internal diameter of 3 mm or more in pediatric patients <5 years old and 4 mm or more in pediatric patients at least 5 years old; a segment with an internal diameter at least 1.5 times larger than that of a nearby segment; or a clear saccular or fusiform extension. A giant coronary aneurysm was defined as an increased internal diameter of 8 mm or more. The secondary study endpoints were 1) the incidence of CALs at 2 weeks after treatment; 2) total fever duration after treatment; and 3) changes in erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), N-terminal pro B-type natriuretic peptide (NT-proBNP) or B-type natriuretic peptide (BNP), and creatine kinase-myocardial band levels before treatment; and 4) when body temperature remained below 37.5℃ for 24 h. Safety was assessed with adverse events, clinical laboratory tests, vital signs, and physical examinations.
All analyses were performed using the statistical software system SAS 9.3 (SAS Institute, Cary, NC, USA).
Categorical data are described as frequencies, percentages, and binomial test p values; continuous data are presented as means, standard deviations, and paired t-test p values. Data obtained from the patients in this clinical trial were classified into safety, intention-to-treat (ITT), and per-protocol (PP) groups for analysis. The safety group comprised all patients who received IVIG-SN, whereas the ITT group included all patients who received IVIG-SN and had a primary efficacy evaluation. The PP group comprised all patients in the ITT group without serious protocol violation. The ITT group was used for efficacy analyses, and an additional analysis was performed on the PP group. The safety group was used for safety analyses. Missing values were not substituted. All statistical analyses were two-tailed, and the significance level was set at 0.05.
The male:female ratio was 0.91:1, and the mean age was 29.11±17.23 months. Four patients had incomplete KD (9%), and the remaining patients had complete KD (Table 1).
Of the 47 patients whose parents or guardians agreed to their participation in this clinical trial, one parent withdrew consent, and one patient did not meet the inclusion criteria and was disqualified during the screening phase. The remaining 45 patients were enrolled. Of the 45 patients, three dropped out without completing the clinical trial: one was disqualified for a violation of inclusion criteria, one was disqualified because normal body temperature was not maintained and none of the clinical symptoms improved within 36 h after the second treatment; and one was disqualified for other reasons. A total of 42 patients completed the clinical trial (Table 2).
To assess the secondary endpoints, the incidence of CALs at 2 and 7 weeks after treatment was examined in the 42 patients in the ITT group. CALs occurred in one patient, which translates to an incidence of 2.38% (95% confidence interval [CI]: 0.06-12.57%). This patient had atypical KD and had a fever for 8 days before treatment. The incidence of CALs in the ITT group was significantly lower than in a previous study on untreated KD,1)6) p=0.0220, 15% vs. 2.38%) (Table 2). The incidence of CALs in the ITT group was lower, although not statistically significantly, than in treated KD (p=0.4361, 5% vs. 2.38%). No patients had refractory KD.
One patient had CALs at 7 weeks after treatment, translating to an incidence of 2.38% (95% CI: 0.06-12.57%) for the primary endpoint. Total fever duration, which was measured from the time of treatment until the body temperature dropped to 37.5℃ or lower, was examined in the ITT group. The average fever duration was 1046.26±1269.88 min (95% CI: 650.54-1441.98 min) or approximately 17.4 h, with a range of 0-5040 min (84 h). Fever duration from the onset of fever until treatment was 5-10 days (mean 6.45±1.30 days). Fever duration was 5 days in 11 patients (25.00%), 6 days in 16 patients (36.36%), and 7-10 days in 17 patients (38.64%). The average ESR was 52.12±27.88 mm/h at baseline and 75.21±28.07 mm/h at admission. ESR increased by 23.10±20.17 mm/h from baseline to admission, which was statistically significant (p<0.0001). The average CRP level was 7.62±6.74 mg/dL at baseline and 3.69±3.41 mg/dL at admission. CRP decreased by 4.02±4.21 mg/dL from baseline to admission, which was statistically significant (p<0.0001). Other laboratory findings are summarized in Table 3.
A total of 44 adverse events were reported in 28 patients (63.64%, 28/44 patients). The investigators evaluated the severity of adverse events as “mild”, “moderate” or “severe” and all 44 adverse events were “mild”. Two patients (4.55%, 2/44 patients) had 3 mild adverse drug reactions that may have had a causal relationship with IVIG-SN. The 3 adverse drug reactions were “skin and subcutaneous tissue disorders”, “blood and lymphatic system disorders” and “investigations” each affecting 1 subject (2.27%, 1 case). There was 1 case each of “eczema”, “anemia” and “increased eosinophil count.” No serious adverse drug reactions occurred (Table 4). The incidence of each type of adverse event is as follows: 11 gastrointestinal disorders (5 diarrhea, 5 vomiting, 2 abdominal pain, 1 mouth hemorrhage, 1 nausea); 6 skin and subcutaneous disorders (2 eczema, 2 rashes, 1 dermatitis, 1 urticaria); 5 infections and infestations (1 bronchitis, 1 hand-foot-and–mouth disease, 1 influenza, 1 nasopharyngitis, 1 upper respiratory tract infection); 4 respiratory, thoracic, or mediastinal disorders (3 epistaxis, 1 cough); 4 general disorders or administration site conditions (2 local swelling, 1 hypothermia, 1 pyrexia); 2 metabolic or nutritional disorders (2 hypophagia, 2 eye disorders (1 conjunctivitis, 1 ocular pain); 1 reproductive system or breast disorder (1 vulvovaginal pruritus); 1 musculoskeletal or connective tissue disorder (1 myalgia); 1 investigational abnormality (1 increased eosinophil count); 1 injury, poisoning, or procedural complication (1 contusion); 1 blood or lymphatic systems disorder (1 anemia); and 1 nervous system disorder (1 headache).
Three events in 2 patients (4.55%) were deemed to be relevant to IVIG-SN, with 2 events being “likely to be relevant” and one being “unclear.” No adverse event resulted in medical intervention or legal action against the manufacturer due to IVIG-SN. A total of 17 adverse events required medication, and they all responded to the drug treatment. There were no serious adverse events during the study.
This clinical trial was conducted to evaluate the efficacy and safety of IVIG-SN (manufactured by Green Cross Corporation) in pediatric patients diagnosed with KD. In the ITT group, the incidence of CALs at 2 and 7 weeks after treatment was 2.38% (1/42 patients). This incidence was lower than the 5% reported for coronary artery complications in untreated patients.1)16) When compared with the CAL incidence of 5% in treated patients, our result had a lower incidence, though the difference was not statistically significant. In a meta-analysis of IVIG results, the incidence of CALs was 10.8% at 30 days and 2.8% at 60 days after the onset of treatment.17) Compared with these previous studies, IVIG-SN was not inferior. In the present study, 31 of the 42 patients had no CALs at any point during the trial. Among the remaining 11 patients, 10 had CALs at admission that disappeared by week 2, and one patient had CALs until the end of the trial.
In the ITT group, investigators assessed the total fever duration from the start of treatment until the body temperature returned to normal. The average fever duration was 1046.26±1269.88 min (approximately 17.4 h), indicating that the fever subsided within 24 h.
Retrospective studies of KD have found that ESR and CRP take time to increase and reach a maximum. Depending on the inconsistency of this increase period, the incidence of CALs may increase. For inflammatory indices, such as CRP, trends and patterns of change are more important than absolute values. Recent studies have reported on other inflammatory factors, such as IL-6, IL-8, and TNF-α.18) In a recent study, elevated segmented WBC (%) and changes in CRP and NT-proBNP levels were used to predict IVIG resistance and CALs.19)
Several IVIG brands are available in Korea and other countries. Each IVIG brand differs in efficacy and adverse effects. Acute kidney injury related to IVIG infusion has been associated with a product containing high sucrose concentrations, and thrombotic events have been associated with a high-dose hyperosmolar product that increases blood viscosity.20) Selecting a product with a higher response rate and avoiding a high infusion rate may prevent IVIG-related complications and lead to better coronary outcomes. With IVIG-SN, 44 adverse events occurred in 28 patients (63.64%, 28/44 patients) including three adverse drug reactions in 2 patients (4.55%, 2/44 patients). All adverse events were “mild”; no serious adverse events occurred during the trial. The adverse drug reactions were “eczema”, “anemia” and “increased eosinophil count” all of which have been reported previously.
All patients showed statistically significant changes in pulse rate and tympanic membrane temperature at the final visit relative to screening. This result could be due to the alleviation of fever symptoms during the trial. Changes in the physical examination were also statistically significant, but they were confirmed to not be significantly related to IVIG administration from a clinical perspective.
The limitations of this study were the small number of patients included and the lack of an active control. Despite these limitations, this trial showed that the incidence of CALs was 2.38% when IVIG-SN was used to treat pediatric patients diagnosed with KD. This was statistically significantly lower than the 5% incidence that was set as the standard in the protocol. This was also lower than a recently reported CAL incidence of 5%, although the difference was not statistically significant. The efficacy of the product was confirmed. No major issues were discovered in terms of safety. IVIG-SN is thought to be safe and effective.
References
1. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986; 315:341–347. PMID: 2426590.
2. Lee DB. Epidemiologic study of Kawasaki disease in Korea (1976-1984). J Cath Coll. 1985; 38:8–19.
3. Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978; 61:100–107. PMID: 263836.
4. Melish ME. Kawasaki syndrome. Pediatr Rev. 1996; 17:153–162. PMID: 8935914.
5. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967; 16:178–222. PMID: 6062087.
6. Taubert KA. Epidemiology of Kawasaki disease in the United States and worldwide. Prog Pediatr Cardiol. 1997; 6:181–185.
7. Yanagawa H, Kawasaki T, Shigematsu I. Nationwide survey on Kawasaki disease in Japan. Pediatrics. 1987; 80:58–62. PMID: 3601519.
8. Kim GB, Han JW, Park YW, et al. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009-2011. Pediatr Infect Dis J. 2014; 33:24–27. PMID: 24064559.
9. Nagashima M, Matsushima M, Matsuoka H, Ogawa A, Okumura N. High-dose gammaglobulin therapy for Kawasaki disease. J Pediatr. 1987; 110:710–712. PMID: 2437278.
10. Ahn SY, Hong SY, Kim NS, Lee HB, Moon SJ, Lee H. Comparison between treatment with aspirin alone and the combined treatment with aspirin and intravenous gamma-globulin in Kawasaki disease. J Korean Pediatr Soc. 1990; 33:1380–1387.
11. Kuribayashi S, Ootaki M, Tsuji M, Matsuyama S, Iwasaki H, Oota T. Coronary angiographic abnormalities in mucocutaneous lymph node syndrome: acute finding and long-term follow-up. Radiology. 1989; 172:629–633. PMID: 2772168.
12. Nakashima L, Edwards DL. Treatment of Kawasaki disease. Clin Pharm. 1990; 9:755–762. PMID: 2242655.
13. Shim SY, Heo MY, Kim HS, Sonh SJ. High-dose intravenous immune globulin retreatment in Kawasaki disease. J Korean Pediatr Soc. 2002; 45:1273–1277.
14. Witt MT, Minich LL, Bohnsack JF, Young PC. Kawasaki disease: more patients are being diagnosed who do not meet American Heart Association criteria. Pediatrics. 1999; 104:e10. PMID: 10390296.
15. Fukushige J, Takahashi N, Ueda Y, Ueda K. Incidence and clinical features of incomplete Kawasaki disease. Acta Paediatr. 1994; 83:1057–1060. PMID: 7841704.
16. Kato H, Ichinose E, Yoshioka F, et al. Fate of coronary aneurysms in Kawasaki disease: serial coronary angiography and long-term follow-up study. Am J Cardiol. 1982; 49:1758–1766. PMID: 7081062.
17. Mori M, Miyamae T, Imagawa T, Katakura S, Kimura K, Yokota S. Meta-analysis of the results of intravenous gamma globulin treatment of coronary artery lesions in Kawasaki disease. Mod Rheumatol. 2004; 14:361–366. PMID: 17143694.
18. Park MJ, Jeon IS, Tchah H, Cho KH, Jung MJ, Choi DY. Predictive indicators of coronary artery complications in Kawasaki disease. Korean J Pediatr. 2009; 52:1161–1166.
19. Cho KH, Kang SJ. Clinically useful predictors of resistance to intravenous immunoglobulin and prognosis of coronary artery lesions in patients with incomplete Kawasaki disease. Korean Circ J. 2014; 44:328–335. PMID: 25278986.
20. Stiehm ER. Lessons from Kawasaki disease: all brands of IVIG are not equal. J Pediatr. 2006; 148:6–8. PMID: 16423589.
Table 1
Demographic characteristics of patients
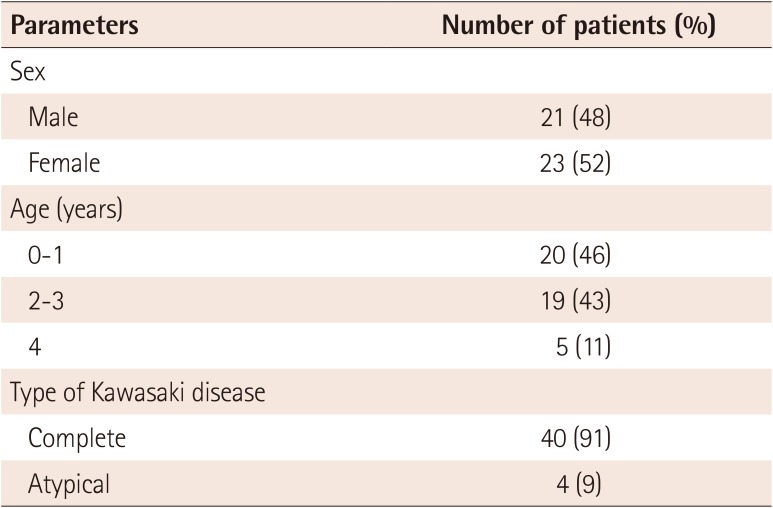
| Parameters | Number of patients (%) |
|---|---|
| Sex | |
| Male | 21 (48) |
| Female | 23 (52) |
| Age (years) | |
| 0-1 | 20 (46) |
| 2-3 | 19 (43) |
| 4 | 5 (11) |
| Type of Kawasaki disease | |
| Complete | 40 (91) |
| Atypical | 4 (9) |
Table 2
Incidence of coronary artery lesions at 7 weeks in the ITT group
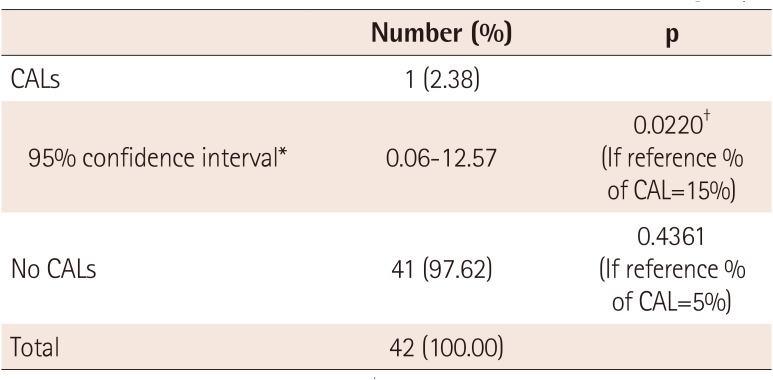
| Number (%) | p | |
|---|---|---|
| CALs | 1 (2.38) | |
| 95% confidence interval* | 0.06-12.57 | 0.0220† (If reference % of CAL=15%) |
| No CALs | 41 (97.62) | 0.4361 (If reference % of CAL=5%) |
| Total | 42 (100.00) |
Table 3
Changes in secondary efficacy variables
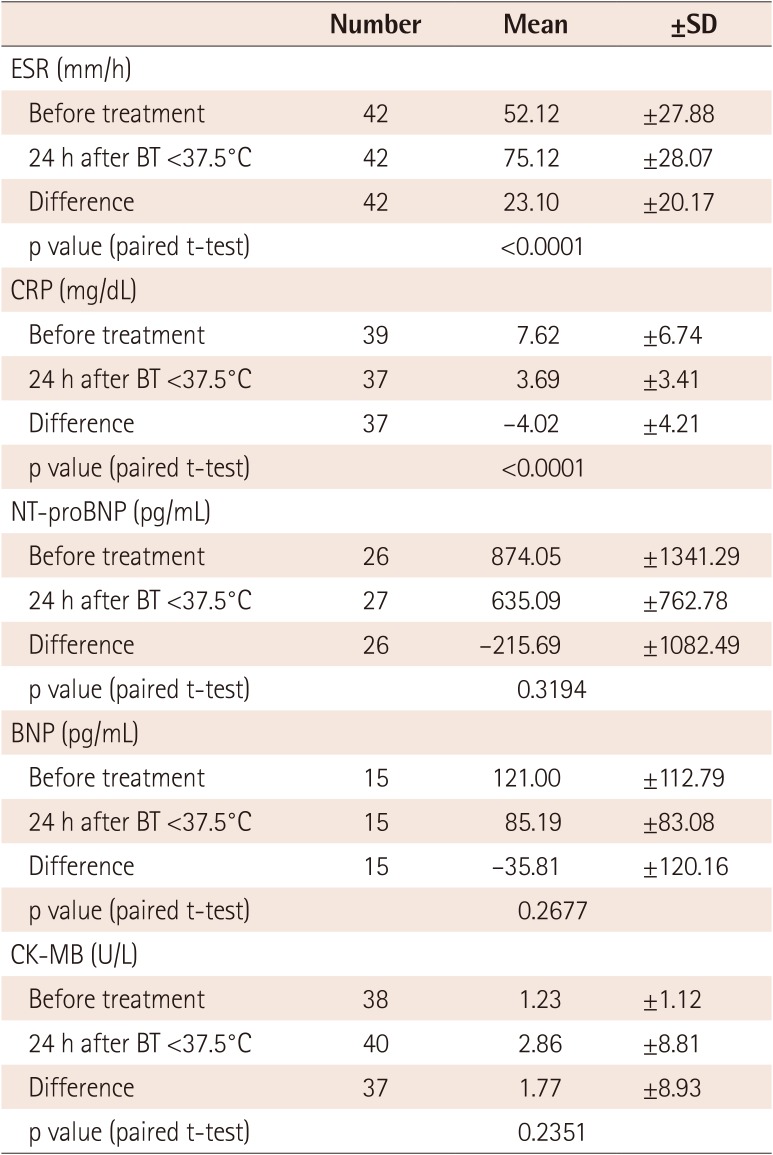
Table 4
Adverse events and adverse drug reactions
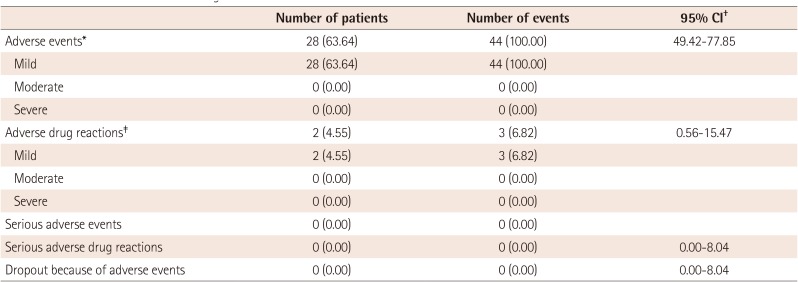
Values are presented as number (%). *The adverse events were not directly related to IVIG-SN. †Fisher's exact confidence Interval. ‡The adverse drug reactions that might related to IVIG-SN included eczema, anemia, and increased eosinophil count. CI: confidence interval, IVIG-SN: interavenous immunoglobulin-SN




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download