Abstract
Right ventricular apical pacing has been a commonly used method for placement of permanent pacemaker, but it is known to be associated with ventricular dyssynchrony and may lead to heart failure. Septal pacing could be an alternative method to improve this complication but the results have been conflicting; hence, other strategies are needed. This case is about a patient with pacing-induced cardiomyopathy who showed much improvement after repositioning the leads to a site different from that of normally paced QRS axis.
For patients who need permanent pacemakers, the right ventricular apex is a commonly preferred site for placement of right ventricular pacing lead because of its easy accessibility. However, recent studies have suggested that right ventricular apical pacing creates abnormal contraction, reduces pump function, and may lead to heart failure. New strategies to overcome these adverse effects, including pacing alternative sites such as the right ventricular outflow tract, His bundle, or septum, have been proposed, but there have been conflicting results.1)2)3)4)5)6)
Since patients show variations in their ventricular anatomy, and there is no definite fluoroscopic landmark between the septum and the apex, fluoroscopic determination of the lead position may not result in physiological activation with normal QRS axis, which is defined as that between -30° and 90°. A study has shown that normally paced QRS axis, rather than radiographically determined septal pacing, leads to better outcomes in preserving the left ventricular function.7)
We present the case of a patient with severe left ventricular systolic dysfunction who, after implanting a permanent pacemaker, showed much improvement with normal QRS axis pacing.
A 76 year-old woman presented to the emergency department with a five-day history of shortness of breath, designated as class III as per the New York Heart Association functional classification. She denied any chest pain, syncope or febrile sense. Her past medical history included complete atrioventricular block, and a permanent pacemaker (DDD, Cylos, Biotronik, Berlin, Deutschland) had been implanted a year earlier.
On physical examination, her pulse rate was regular and measured 100 bpm; blood pressure was 140/80 mmHg and body temperature 36.5℃. Chest auscultation revealed clear breathing sounds, and cardiovascular examination revealed normal heart sounds with no added sounds or murmurs. There was no pedal edema. Chest X-ray showed marked cardiomegaly, and electrocardiogram (ECG) showed atrial-sensed ventricular-paced rhythm with a rate of 105/min. The patient was dependent on ventricular pacing (>99% pacing). The laboratory data for complete blood count and blood chemistry were in the normal range. The cardiac markers, including CK-MB and troponin-I, were also in the acceptable normal range (2.86 ng/mL and 0.077 ng/mL, respectively). However, the pro-brain natriuretic peptide was elevated to 13095 pg/mL.
The patient was transferred to a general medical ward. The echocardiogram done prior to implantation a year earlier had shown normal left ventricular ejection fraction (LVEF) of 67%, and the coronary angiogram had shown minimal disease, with a diffuse eccentric 30% stenosis at the mid right coronary artery, and normal left coronary arteries. However, the echocardiogram done at present, showed diffuse global hypokinesia and dilatation of left ventricle with decreased ejection fraction of 31%.
The investigation and medical review of the patient was done to decipher the possible causes of the left ventricular dysfunction, including myocardial infarction, severe valvular heart disease, uncontrolled hypertension, infection, thyrotoxicosis, and drugs; however, all these conditions were all ruled out. Since there was no proven alternative cause to induce the cardiomyopathy, the patient was diagnosed as pacing-induced cardiomyopathy, which was defined as a ≥10% decrease in LVEF, with value of LVEF<50%.
The management of heart failure was done with medical therapy, which included angiotensin converting enzyme inhibitors, beta blockers and diuretics. We also decided to undertake the procedure of pacing lead replacement to prevent worsening of the left ventricular dysfunction by abnormal axis right ventricular pacing. We dissected the site which the distal part of the right ventricular lead was adhered to, and by unscrewing and pulling manually, the lead came off from the ventricular wall with ease. The lead was replaced to the septum (Fig. 1A and B) at the point showing normal QRS axis at ECG monitor (Fig. 2A and B).
The post-operative course was uneventful. A week after normal QRS axis pacing, her echocardiogram showed improvement of the left ventricular systolic function with ejection fraction of 42%. Her symptoms showed much improvement, and she was discharged. She was asked to come for regular follow-ups at our out-patient department.
There were significant changes of ECG and chest X-ray of the patient at 6 month follow-up (Figs. 1C and 2C). Echocardiogram was done a year later at our out-patient department, and further improvement of the systolic function up to LVEF of 55%, and a markedly decreased left ventricular size were noted (left ventricular end-diastolic dimension was 58 mm and 45 mm, left ventricular end-systolic dimension was 42 mm and 28 mm, at admission and at 1 year follow-up, respectively; left ventricular end-diastolic volume was 97 mL and 35 mL, left ventricular end-systolic volume was 67 mL and 16 mL, at admission and at 1 year follow-up, respectively).
For patients with symptomatic bradycardia due to sinus node dysfunction and atrioventricular block, implanting a pacemaker is the only appropriate management in current practice. Right ventricular apex is a common site for placement of the ventricular pacing lead because it is easy to reach and yields stable mechanical positions.
However, studies and reports have shown that chronic right ventricular apical pacing is associated with ventricular dyssynchrony, left ventricular systolic dysfunction and adverse clinical events.1)2)3)4)5)6) In a few studies, the prevalence of pacing-induced cardiomyopathy is suggested to be 9% at 1 year after pacemaker implantation, and increases up to 15% after 25 years of chronic RV pacing.6)8) There is currently no exact data of reversibility of pacing-induced cardiomyopathy and the best treatment options to overcome these adverse effects, except some case reports.9)10)11)12) New strategies have been proposed, and clinical trials are ongoing.
Alternative pacing sites to replace the right ventricular apex, such as His bundle, RV outflow tract and septum, have been suggested. His bundle pacing preserves the native ventricular activation sequence and seems to be most ideal, but technically challenging, procedure. RV outflow tract and septal pacing seems to yield positive data on cardiac functions, but there are conflicting evidences on the clinical benefits (such as exercise capacity or quality-of-life scores), and survival.13)14)15)
This case shows a reversal of pacing-induced left ventricular systolic dysfunction after replacing the ventricular pacing lead from abnormally paced QRS axis to normally paced QRS axis, in a patient with initially preserved left ventricular ejection fraction.
The pacing-induced cardiomyopathy was observed 1 year after the pacemaker implantation in our case, and it seemed that rapid deterioration of systolic function was ongoing. Apart from the medical treatment for heart failure, other intervention would be needed to preserve the patient's cardiac function since she was diagnosed with pacing-induced cardiomyopathy. Cardiac resynchronization therapy was considered but was not on absolute indication at that time and could not be put into practice because of the other factors, such as insurance problem in this case.
Thus, our decision was to replace the pacing lead to a different site. We observed an acute improvement 1 week after pacing the alternate site with normal QRS axis, and the heart failure eventually resolved within a year, with the patient regaining normal systolic function.
The patient also underwent medical treatment for heart failure the remaining follow-up period. We believe that could also contribute to improvement of the left ventricular systolic function, and this factor could be the limitation of our case report. Still, the acute improvement seen immediately, 1 week from the replacement, could also reflect a meaningful outcome.
Further studies on this phenomenon and proper management are required and expected to achieve positive outcomes in later days, for those who are experiencing heart failure as our patient.
Figures and Tables
Fig. 1
Radiographic changes of the patient before and after normal QRS axis pacing. (A) Initial chest X-ray at admission shows implanted pacemaker with marked cardiomegaly. (B) Chest X-ray of the patient immediate post lead repositioning with resultant right ventricular lead placed in septum. (C) Chest X-ray of the patient 1 year after normal QRS axis pacing showing significant improvement of cardiomegaly.
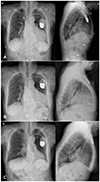
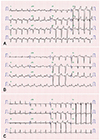
Fig. 2
Electrocardiogram (ECG) changes of the patient before and after normal QRS axis pacing. The QRS duration was 144, 138, and 96 ms at admission, immediate post lead repositioning, and 6 months later, respectively. (A) Initial ECG at admission showing abnormal QRS axis. (B) ECG of the patient immediately after post lead repositioning to achieve normally paced QRS axis. (C) ECG of the patient 6 months after the lead repositioning.
References
1. Tse HF, Yu C, Wong KK, et al. Functional abnormalities in patients with permanent right ventricular pacing: the effect of sites of electrical stimulation. J Am Coll Cardiol. 2002; 40:1451–1458.
2. Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003; 107:2932–2937.
3. Thackray SD, Witte KK, Nikitin NP, Clark AL, Kaye GC, Cleland JG. The prevalence of heart failure and asymptomatic left ventricular systolic dysfunction in a typical regional pacemaker population. Eur Heart J. 2003; 24:1143–1152.
4. Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation. 2006; 113:2082–2088.
5. Lieberman R, Padeletti L, Schreuder J, et al. Ventricular pacing lead location alters systemic hemodynamics and left ventricular function in patients with and without reduced ejection fraction. J Am Coll Cardiol. 2006; 48:1634–1641.
6. Yu CM, Chan JY, Zhang Q, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009; 361:2123–2134.
7. Kim SH, Oh YS, Nam GB, et al. Paced QRS axis as a predictor of pacing-induced left ventricular dysfunction. J Interv Card Electrophysiol. 2014; 41:223–229.
8. Dreger H, Maethner K, Bondke H, Baumann G, Melzer C. Pacing-induced cardiomyopathy in patients with right ventricular stimulation for >15 years. Europace. 2012; 14:238–242.
9. Te CC, Stavrakis S, Lozano P, Reynolds D. Apparent acute reversible right ventricular pacing-induced left ventricular dysfunction. J Cardiovasc Electrophysiol. 2013; 24:224–226.
10. Fung JW, Zhang Q, Yip GW, Yu CM. Reversible left ventricular dyssynchrony and heart failure induced by right ventricular pacing. Int J Cardiol. 2009; 134:117–119.
11. Vanagt WY, Prinzen FW, Delhaas T. Reversal of pacing-induced heart failure by left ventricular apical pacing. N Engl J Med. 2007; 357:2637–2638.
12. Ouali S, Azzez S, Kacem S, et al. Acute left ventricular dysfunction secondary to right ventricular septal pacing in a woman with initial preserved contractility: a case report. J Med Case Rep. 2011; 5:524.
13. Victor F, Leclercq C, Mabo P, et al. Optimal right ventricular pacing site in chronically implanted patients: a prospective randomized crossover comparison of apical and outflow tract pacing. J Am Coll Cardiol. 1999; 33:311–316.
14. Stambler BS, Ellenbogen K, Zhang X, et al. Right ventricular outflow versus apical pacing in pacemaker patients with congestive heart failure and atrial fibrillation. J Cardiovasc Electrophysiol. 2003; 14:1180–1186.
15. Cano O, Osca J, Sancho-Tello MJ, et al. Comparison of effectiveness of right ventricular septal pacing versus right ventricular apical pacing. Am J Cardiol. 2010; 105:1426–1432.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download