Abstract
Drug-eluting stent (DES) implantation is currently the standard treatment for various types of coronary artery disease. However, previous reports indicate that stent fractures, which usually occur after a period of time from the initial DES implantation, have increased during the DES era; stent fractures can contribute to unfavorable events such as in-stent restenosis and stent thrombosis. In our present report, we describe two cases of zotarolimus-eluting stent fracture: one that was detected six hours after implementation, and the other case that was detected immediately after deployment. Both anatomical and technical risk factors contributed to these unusual cases of immediate stent fracture.
Stent fractures have recently become an important concern in the medical community due to their potential association with serious conditions such as in-stent restenosis (ISR) and stent thrombosis after drug-eluting stent (DES) implantation.1) Most currently reported stent fractures are found in lesions implanted with sirolimus-eluting stents (SES), likely due to the inherent platform material and design of such stents.2) Furthermore, while the exact timing of fractures following stent implantation remains unclear, nearly all reports identify fractures after a time period of follow-up for patients after stenting, suggesting a delayed complication timeline.1)3)4)5) In this report, we present two unusual cases of immediate stent fracture that occurred after implantation of new-generation DES, a zotarolimuseluting stent (ZES).
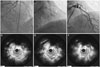
A 62-year-old man, who had undergone cardiac transplantation for advanced heart failure, was referred to our catheterization laboratory for a regular surveillance coronary angiogram and concomitant intravascular ultrasound (IVUS) imaging. After identifying a normal right coronary angiogram (Fig. 1), the clinician attempted to insert a 0.014-inch BMW (Abbott Vascular, Melno Park, CA, USA) wire for IVUS evaluation. However, resistance was quickly met, and the wire failed to pass through the proximal portion of the right coronary artery (RCA). A subsequent angiogram revealed a spiral luminal filling defect from the proximal to the distal RCA indicating coronary dissection and Thrombolysis in Myocardial Infarction (TIMI) 2 flow (Fig. 1). The ST segment elevation appeared in lead II and III on electrocardiography (ECG) monitoring, while hemodynamic parameters were stable. Urgent bailout stenting was performed: the RCA was engaged with 8 Fr guiding catheter (JR 4.0, Cordis, Bridgewater, NJ, USA); a BMW wire was inserted using a 1.8 Fr Finecross (Terumo Medical, Tokyo, Japan) micro-guiding catheter; the true lumen was confirmed by IVUS-virtual histology; and three ZES stents (Resolute Integrity 2.75×30 mm, 3.0×38 mm, 3.5×30 mm, Medtronic, Santa Rosa, CA, USA) were deployed in order from distal to proximal at 12, 16, and 16 atm, respectively, with overlap between adjacent stents. Although the right ventricular branches were compromised, final RCA angiography showed optimal angiographic results with TIMI 3 flow; ECG abnormalities eventually returned to baseline.
During close clinical observation, a follow-up ECG, performed six hours after the procedure, showed new ST segment elevation of 2 mm in leads V 1-2, and a blood test revealed elevated levels of troponin I (29.834 ng/mL, normal level <1.5 ng/mL). The patient was hemodynamically stable and did not complain of chest pain, likely due to anatomical denervation of the allograft during transplantation. Considering the occurrence of the previous adverse event, a follow-up coronary angiography was performed and showed a complete linear transverse fracture of the previously inserted stent (Fig. 1). The fracture site was close to the stent overlap, at the distal edge of the first proximal stent; hinge motion was noted during the cardiac cycle. Although there was evidence of some misalignment observed, the two fractured segments still maintained contact without definite displacement. Since there was no evidence of obstruction at that time and TIMI 3 flow was observed, the procedure was terminated after ascertaining the left coronary artery was normal. Echocardiography showed new right ventricular dysfunction, but the patient remained hemodynamically stable without any discomfort.
The patient was discharged from the hospital three days later with standard dual antiplatelet drugs and immunosuppressive agents (tacrolimus, prednisone and mycophenolate mofetil). After 12- months of clinical follow-up without further issues, routine surveillance coronary angiography was performed. Previous stents were deemed stable without restenosis at the original site of stent fracture.
A 75-year-old man was admitted with a history of effort-related chest pain persisting for two years. Prior to being admitted, his chest pain increased in intensity and frequency despite taking appropriate medications. The patient had a 10-year documented history of hypertension and was an ex-smoker. An ECG showed a left ventricular hypertrophy pattern without ST segment change. A Thallium-201 myocardial perfusion scan showed severe reduction of tracer uptake in the septum and anterior walls. Coronary angiography revealed diffuse eccentric stenosis in the left anterior descending artery (LAD) (Fig. 2). A minimally invasive direct bypass surgery was planned because the lesion was long, originated from the ostium of the LAD, and was accompanied by very heavy calcification: this indicated that the patient was not suitable for coronary intervention. However, the patient also had comorbid psychological issues including anxiety disorder and claustrophobia, which were not ameliorated by professional psychotherapy; the patient strongly refused surgery. Considering the patient's extant physical and psychological issues, coronary intervention was a suboptimal option for him.
The LAD was engaged with a 7 Fr guiding catheter (JL 4.0, Cordis, Bridgewater, NJ, USA), predilatation was performed with a TREK 2.5×20 mm balloon (Abbott Vascular, Santa Clara, CA, USA), and two ZES stents (Resolute Integrity 2.5×30 mm, 3.0×34 mm, Medtronic, Santa Rosa, CA, USA) were deployed at 9 atm in an overlapping manner. Additional high-pressure postdilation with a Nimbus Salvo non-compliant balloon (3.0×17mm, Clearstream Tech, Wexford, Ireland) at 16 atm and Empira non-compliant balloon (4.0×10 mm, Cordis) at 24 atm was performed.
During the final angiography, a gap was found in the proximal portion of the second distally implanted stent, which was indicative of a fracture; rotational fluoroscopic images showed consistent findings (Fig. 2). Similar to the first reported case, the fracture site was close to the overlap at the distal edge of the first proximal stent, and hinge motion was also noted during the cardiac cycle. IVUS with an automated pullback speed of 0.5 mm/s revealed partial absence of the stent struts corresponding to the area of the stent fracture (Fig. 2). Except for the stent fracture, IVUS showed good results with complete stent apposition and sufficient expansion. We opted not to perform any further interventions, and the patient was discharged with antianginal medications including triple antiplatelet agents with the regimen of aspirin, clopidogrel and cilostazol. At the 6-month clinical follow-up point, the patient was asymptomatic for chest pain or dyspnea and exercise stress tests were negative.
The overall reported incidence of stent fracture approximately ranges between 1% and 8% in the current era of DES implantation; the figure varies widely based on the method of stent fracture detection, the type of stent implanted, and the population studied.6) However, the timing of stent fracture after implantation and changes in stent fracture incidence over time are not well understood phenomenon. Stent fracture is generally posited to occur after a period of time has passed from the initial stent implantation. Indeed, most studies indicate that stent fractures are detected in clinically driven follow-up imaging ranging from months to years after stenting.1)2)3)4)5) In contrast, cases of immediate stent fracture are scarce, at least those reported in the extant medical literature.7)8)
Our present findings importantly evidence that stent fractures can occur in the immediate period after angioplasty, particularly in patients with predisposing risk factors. In our first case, creating a full metal jacket in the RCA with multiple overlapping long stents was unavoidable. In general, the RCA is more dynamic than other epicardial vessels, meaning that more flexion points exist in certain segments during the cardiac cycle.9) A stent in this location can be subjected to repetitive distorting forces, which can cause a fracture resulting from mechanical fatigue. In addition, longer stents are prone to higher radial forces during vessel contraction and bending during the systolic cycle.10) Moreover, stent overlap increases local axial rigidity and decreases stent conformability, which can result in hinge motion near the overlap, ultimately resulting in fracture.11)
Additional risk factors for stent fracture were observed in our second case. Diffuse calcification can alter the distribution of stress within the vessel wall.12) The subsequent use of a high-pressure balloon in these calcified areas can damage the stent structure by augmenting the strain difference within the stent. We believe these anatomical and technical factors jointly contributed to the rare occurrence of early stent fracture in this patient.
Extant observational studies have indicated that most cases of stent fracture occur with Cypher SES (Cordis).2) In such cases, the fracture can be attributed to the platform material and design of SES, with its relatively thick, rigid, and closed-cell structure that leads to a loss of stent conformability and renders it less deformable in response to dynamic loading imposed by cardiac movement. ZES, a new-generation DES, was utilized in our two current cases and has potential advantages over SES due to the presence of a thin-strut cobalt-chrome alloy platform and an open-cell design.13) However, these putative advantages, related to the stent's improved construction, were ultimately unable to prevent fracture: this result suggests that the contribution of the risk factors mentioned above may have played a more significant role in the fracture.
Evidence suggests that stent fracture increases the probability of stent thrombosis and ISR, leading to a higher rate of target lesion revascularization.1)2)3)4)5) Theoretically, drug delivery at the fracture site can be poor and the vessel can receive higher mechanical irritation due to fractured struts, causing smooth muscle cell proliferation and impaired endothelial coverage.1) However, not all stent fractures are associated with clinical sequelae; this diverging result may be partially explained by the type of stent implanted, the duration or differences related to medication use, and the overall degree of stent fracture. Currently, owing to the lack of clinical understanding regarding the importance of contributing risk factors, there is no uniform consensus regarding the best treatment method for stent fracture.11) Also, it is further unknown whether a preventive treatment strategy of uncomplicated stent fracture, as demonstrated in our cases, may benefit the patient. Management of the fracture should thus be individualized, according to the presence of ischemia induced by restenosis, the type of stent fracture, and the presence of factors that predict possible recurrence.14)15) In our first case, despite a complete stent fracture, additional treatment was not performed; this was due to the absence of flow disruption and the fact that the stent fracture was caused mainly by non-modifiable factors. In contrast, stent overexpansion, which is a modifiable factor, was arguably the main reason for the fracture in our second case. Although restenting with causion may have been possible in this case, the degree of fracture was low enough to warrant a more conservative approach of follow-up without further treatment.
Overall, our current cases highlight that stent fracture can occur immediately after stenting, even after ZES implantation. Physicians should consider all risk factors for stent fracture during angioplasty to minimize the risk of further complications.
Figures and Tables
Fig. 1
Serial coronary angiograms in case 1. A: normal right coronary angiogram. B: right coronary angiogram showing intraluminal spiral-shaped filling defect from proximal to distal artery. C: fluoroscopic imaging shows complete linear transverse fracture (arrow) of the implanted stent. D: there is no evidence of flow obstruction at the fracture site. Note the compromised right ventricular branches in B and D.

Fig. 2
Representative coronary angiographic and IVUS findings in case 2. A: AP caudal view of coronary angiogram shows diffuse eccentric stenosis from ostium to mid-portion of left anterior descending artery. B: fluoroscopic image shows gap (arrow) at the border of distally implanted stent suspicious of stent fracture. C: small indentation (arrow) is noted at the site of gap during angiogram. D, E, and F: serial IVUS images revealed partial absence of the metallic stent strut corresponding to the area of the gap. IVUS: intravascular ultrasound.
References
1. Kuramitsu S, Iwabuchi M, Haraguchi T, et al. Incidence and clinical impact of stent fracture after everolimus-eluting stent implantation. Circ Cardiovasc Interv. 2012; 5:663–671.
2. Chakravarty T, White AJ, Buch M, et al. Meta-analysis of incidence, clinical characteristics and implications of stent fracture. Am J Cardiol. 2010; 106:1075–1080.
3. Aoki J, Nakazawa G, Tanabe K, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007; 69:380–386.
4. Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc Interv. 2007; 69:387–394.
5. Kim HS, Kim YH, Lee SW, et al. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. 2009; 133:354–358.
6. Alexopoulos D, Xanthopoulou I. Coronary stent fracture: how frequent it is? Does it matter? Hellenic J Cardiol. 2011; 52:1–5.
7. Amico F, Geraci S, Tamburino C. Acute coronary syndrome due to early multiple and complete fractures in sirolimus-eluting stent: a case report and brief literature review. Catheter Cardiovasc Interv. 2013; 81:52–56.
8. Smaldone C, Bacà M, Niccoli G. A case of early drug-eluting stent fracture. J Invasive Cardiol. 2010; 22:E186–E188.
9. Chhatriwalla AK, Unzek S, Kapadia SR. Recurrent stent fracture in the right coronary artery. Clin Cardiol. 2010; 33:E70–E72.
10. Sianos G, Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004; 61:111–116.
11. Mohsen MK, Alqahtani A, Al Suwaidi J. Stent fracture: how frequently is it recognized? Heart Views. 2013; 14:72–81.
12. Halwani DO, Anderson PG, Brott BC, Anayiotos AS, Lemons JE. The role of vascular calcification in inducing fatigue and fracture of coronary stents. J Biomed Mater Res B Appl Biomater. 2012; 100:292–304.
13. Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013; 368:1558.
14. Popma JJ, Tiroch K, Almonacid A, Cohen S, Kandzari DE, Leon MB. A qualitative and quantitative angiographic analysis of stent fracture late following sirolimus-eluting stent implantation. Am J Cardiol. 2009; 103:923–929.
15. Doi H, Maehara A, Mintz GS, et al. Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am J Cardiol. 2009; 103:818–823.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download