Abstract
Background and Objectives
This study aimed to investigate the clinical characteristics of infantile Kawasaki disease (KD), and to evaluate early diagnostic features of KD in febrile infants.
Subjects and Methods
We retrospectively reviewed the medical records of 64 KD patients from January 2010 to October 2014. There was an analysis of the clinical, laboratory data of the infants versus children groups. Furthermore, the clinical and laboratory data of infantile KD patients were compared with 16 infants who were admitted for other acute febrile diseases.
Results
A total of 64 patients with KD were identified; 20 (31.3%) were infants; 44 (68.8%) were >1 year old children. Incomplete KD was much more common in infants (n=13, 65.0%) than in children group (n=14, 31.8%) (p=0.013). The infants were characterized by significantly higher rates of inflammatory changes at the Bacille Calmett-Guérin (BCG) inoculation site (p<0.001), but lower rates of changes in the extremities (p=0.029) and cervical lymphadenopathy (p=0.006). The serum levels of platelet after 1 week (p=0.005), C-reactive protein (p=0.038), and N-terminal pro-brain natriuretic peptide (NT-proBNP) (p=0.026) were all significantly higher in the infants group. Comparing the infants with KD versus the other acute febrile diseases, there were significantly higher serum levels of erythrocyte sedimentation rate (p=0.002), C-reactive protein (p=0.046) and NT-proBNP (p=0.001) for the infants with KD group.
Kawasaki disease (KD) is an acute systemic vasculitis that primarily affects the coronary arteries in young children.1) The KD infants have a higher risk of coronary artery abnormalities, which are the most common and one of the most life threatening complications.2)3) There is no accurate diagnostic marker for KD, so the diagnosis is based on a combination of characteristic clinical findings.4) Incomplete KD is defined as the presence of fewer than four of those principal clinical findings with coronary artery lesions, according to Japanese guidelines.5) However, the criteria are too restrictive to diagnose incomplete KD without coronary artery lesions. Not all the KD patients have coronary artery abnormalities, and it is too late to treat when there is a development of coronary artery abnormalities. Incomplete KD was defined as the presence of less than four classic presentations with supplemental laboratory criteria, regardless of coronary artery lesions, according to the American Heart Association (AHA).1)
In infants, incomplete KD is much more common than children. The diagnosis can be difficult and the treatment might be delayed in infantile KD patients, who have much more risk of coronary artery abnormalities. Thus, additional diagnostic tools are necessary for the early diagnosis of KD in infants. Recently, erythema and induration at the Bacille Calmette-Guérin inoculation site (BCGitis) are considered as significant diagnostic feature in infants with KD.6) Furthermore, the serum level of N-terminal pro-brain natriuretic peptide (NT-proBNP) is also a useful diagnostic marker of KD and risk factor of coronary artery lesions.7) These additional features may help to differentiate and identify KD in febrile infants.
This study was aimed to investigate clinical characteristics of infantile KD, as well as to evaluate the early diagnostic features of KD in febrile infants.
We retrospectively reviewed the medical records of 64 patients with KD who were admitted to the Dongguk University Gyeongju Hospital, Korea, between January 2010 and October 2014. Patients were divided into 2 groups on the basis of their age at diagnosis: infants (≤12 months, n=20) and children (>12 months, n=44). There was an analysis of the clinical characteristics, laboratory findings, and echocardiographic data.
Complete KD was defined as fever for ≥5 days and at least 4 of the 5 principal criteria. Incomplete KD was defined as patients with 4 or fewer of principal criteria, regardless of coronary artery abnormalities. Furthermore, incomplete KD was diagnosed only when those patients showed a markedly elevated platelet and desquamation of the extremities at the convalescent phase. The patients with typical clinical and laboratory features, including BCGitis, were considered to be complete or incomplete KD, even if they had fever less than 5 days.
An echocardiographic examination was performed 2 or 3 times using ALOKA (Aloka ProSound SSD-5000 HD, Olympus, Tokyo, Japan) during the acute phase and the subacute phase (4 weeks later after the onset of disease). The same pediatric cardiologist performed all of the echocardiograms. The diameter of three major coronary arteries were measured: left main coronary artery (LMCA), left anterior descending artery (LAD), right coronary artery (RCA). The measured diameters of the coronary arteries were converted to a z score, as based on the study by Olivieri et al.8) An echocardiogram is considered positive if any of the following 3 conditions were met: the z score of the LAD or RCA≥2.5, coronary arteries meet the criteria for aneurysms9) as promulgated by the Japanese Ministry of Health, or ≥3 other suggestive features exist, including perivascular brightness, lack of tapering, decreased left ventricular function, mitral regurgitation, pericardial effusion, and a z score of LAD or RCA of 2-2.5.1)
The following laboratory data were obtained serially: white blood cell count (WBC), hemoglobin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), liver function tests, and NT-proBNP. Pyuria was defined as urine WBC≥10/HPF.
Furthermore, the demographic and laboratory data of infantile KD patients were compared with 16 infantile patients with other acute febrile diseases who had at least 5 days of fever duration.
The data was presented as mean±standard deviation. All statistical analyses were done using SPSS, version 18 (IBM, New York, United States). The p smaller than 0.05 were considered statistically significant. The statistical analysis relied on the Student's t-test and the chi-square test to compare the two groups. A receiver operating characteristic curves analysis was performed, on NT-proBNP, to predict those infants with KD.
This study was approved by the Institutional Review Board of Dongguk University Gyeongju Hospital, Korea, and the requirement for individual consent was waived for the retrospective study.
Of the 64 patients in the study, 20 (31.3%) were 12 months of age or younger and 44 (68.8%) were older than 12 months. The rate of incomplete KD was higher in the infants (n=13, 65.0%) than in the children (n=14, 31.8%) (p=0.013). The male to female ratio was 3.0 in the infants and 1.0 in the children. The mean age at diagnosis was 5.7 months in the infants and 38.1 months in the children (Table 1).
The duration of fever before intravenous immunoglobulin (IVIG) treatment was shorter in the infants (5.00 days) than in the children (5.41 days) (p=0.203). Furthermore, the total duration of fever was a little shorter in the infants (6.45 days) than in the children (6.48 days) (p=0.481) The infantile group was characterized by significantly lower rates of changes in the extremities (p=0.029) and cervical lymphadenopathy (p=0.006) and higher rates of inflammatory change at the Bacille Calmette-Guérin (BCG) inoculation site (p<0.001); whereas no difference was noted in changes of the lips and oral cavity, conjunctival injection, and polymorphous exanthema (Table 1).
Several laboratory data showed differences between the two groups. The levels of hemoglobin were lower in the infants (10.9±1.0 g/dL) than the children (12.1±1.0 g/dL) (p<0.001). However, age-adjusted anemia was not significantly different between the two groups. The platelet counts after 1 week were significantly higher in the infants (740450±316754/µL) than the children (562372±194465/µL) (p=0.005). Although both acute phase reactants, the ESR and CRP levels, were elevated, only the CRP level was significantly higher in the infants (10.9±12.1 mg/dL) than in the children (6.9±5.5 mg/dL) (p=0.038). Also, the NT-proBNP level was significantly higher in the infants (2442±1866 pg/mL) than the children (945±1151 pg/mL) (p=0.026). There were no significant differences in the levels of albumin, WBC, aspartate aminotransferase, alanine aminotransferase between the two groups. Pyuria was significantly higher in the infants (n=12, 60.0%) than the children (n=12, 28.6%) (p=0.012) (Table 2).
On 2-D echocardiography, the infants (n=3, 15.0%) had higher incidence rates of coronary artery abnormalities than the children (n=4, 9.1%). But there was no significant difference between two groups (p=0.483). IVIG treatment was given to all patients. The IVIG retreatment rate was much higher in the infants (n=4, 20.0%) than the children (n=1, 2.3%) in cases of refractory KD (p=0.014) (Table 3).
In comparing infantile KD with other acute febrile diseases in this study, there were no significant differences in the mean age, male-to-female ratio, and the total fever duration. However, in the occurrence of BCGitis, the infants with KD showed 80%, whereas, there was no case of BCGitis in infants without KD. Furthermore, there were significantly higher serum levels of ESR (63±28 vs. 35±25 mm/hr, p=0.002), CRP (10.8±12.2 vs. 5.0±5.0 mg/dL, p=0.046), and NT-proBNP (2442±1886 vs. 462±695 pg/mL, p=0.001) in the infants with KD. But there was no significant difference in the WBC counts between the two groups (14441±4957 vs 14 891±6 586 /µL, p=0.411) (Table 4).
Concerning the receiver operating characteristic curves analysis (the area under the curve), for the overall ability of the test to discriminate between those infants with the KD and without KD, the data were as follows: serum WBC (cells/µL), 0.486; ESR (mm/hr), 0.910; CRP (mg/dL), 0.653, and NT-proBNP (pg/mL), 0.889. Therefore, the serum level of ESR, CRP and NT-proBNP were considered to be good predictors for KD in febrile infants. Each an optimal cut-off value of ESR (30 mm/hr), CRP (3.0 mg/dL), and NT-proBNP (700 pg/mL) yielded a sensitivity of 89%, 74%, and 78%, a specificity of 38%, 38%, and 88%, a positive predictive value of 63%, 58%, and 78% and a negative predictive value of 75%, 55%, and 88%, all respectively, for predicting KD in febrile infants.
KD is an acute, self-limited vasculitis of unknown etiology that occurs predominantly in infants and young children.1) It was first described in Japan in l967 by Kawasaki.10) In a long-term follow-up study, coronary artery abnormalities, which are the most common and one of the most life threatening complications, are developed in up to 25% of untreated KD children and may lead to myocardial infarction, ischemic heart disease, and sudden death.11) Infants have high risk of coronary artery abnormalities and commonly present incomplete KD.2)3) Incomplete KD can be suspected in patients who have fever for at least 5 days with no other explanation but have fewer than four of the classic diagnostic clinical criteria.1)4) Those infants need the treatment mostly, but they are the ones who are the most difficult to diagnose KD. Thus, additional clinical features and laboratory findings are necessary to early diagnosis of KD in infants. Up to the present time, there has been a lot of effort to early diagnose KD by many physicians, and some progress has been made.
The proportion of incomplete KD was reported to be inversely to age, which means incomplete KD was more common in young infants (76%) than in older children (15%).12)13)14)15) In this study, the rate of incomplete form was also significantly higher in infants than children. The higher incidence of the incomplete form in infants are postulated by several immunologic causes: (1) superantigen neutralization by maternal transplacental antibodies, (2) cross-reactivity of antibodies generated by frequently active immunization, and (3) weak vasculitis phenomenon caused by inadequate immunity response.12)
In the classic diagnosis of KD, the fever must persists ≥5 days. However, according to the AHA diagnostic guidelines, experienced clinicians can make a diagnosis before day 5 of the fever in the presence of classic features.4) Also, the Japanese diagnostic guidelines noted that those with fever subsided before the fifth day in response to therapy can be included in KD.5) In this study, the mean duration of the fever was 5.00 days in infants before treatment with the IVIG. We believe that early treatment with IVIG resulted in a low incidence of coronary artery abnormalities, compared with other reports.11)16) Hence, physicians should not hesitate to begin KD treatment for highly suspicious patients with typical clinical and laboratory features, even if they have fever less than 5 days.
Of the classic KD diagnostic criteria, incidence of changes in extremities and cervical lymphadenopathy was relatively lower than other findings in infant.3)12)15) In this study, the incidence of these two findings was more significantly lower in infants than in children. Cervical lymphadenopathy (20.0%) was the lowest, and changes in extremities (50.0%) was the second lowest in infants. The lower incidence of these two findings in infants implies that the incomplete form is more common in infants. Therefore, other clinical and laboratory features are critical to diagnose infants with incomplete KD.
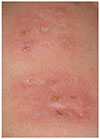
Recently, there have been studies that erythema and induration at the BCG inoculation site is frequently observed in KD (Fig. 1).17)18)19)20) It is believed that those are a nearly specific sign of KD, and some authors have even considered it as a finding of KD.6)19)21) Korea is considered a highly endemic country of tuberculosis and has been conducting routine BCG vaccination to neonates. In countries using universal BCG vaccination like Korea, the BCGitis should be considered as a significant finding, as well as classic diagnostic criteria, because this rare phenomenon is commonly observed in KD and scarcely seen in other conditions. The reported incidence of BCGitis is >70% in those aged 3 to 20 months and 85.3% in infants.6)19) In this study, the incidence was 80.0% in infants, which is compatible with the result of aforementioned studies, and 18.2% in children showing a steeply decreasing pattern with age. Because this rare sign is more common in infants, it can be a useful diagnostic tool for identifying incomplete KD in infants. Physicians who diagnose infantile KD should exam thoroughly, including a BCG injection site.
Although laboratory tests are not included in the principal clinical criteria, their importance should not be overlooked. There are a number of laboratory tests which are helpful for diagnosis of KD, such as leukocytosis, elevated ESR and CRP, anemia, thrombocytosis (after 7 days), elevated serum transaminase, and sterile pyuria.1) The AHA provided a diagnostic algorithm for incomplete KD that included two acute phase reactants (ESR≥40 mm/hr, CRP≥3.0 mg/dL) and 6 supplemental laboratory criteria (albumin≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase, platelets after 7 days ≥450000/mm3, white blood cell count≥15000/mm3, and urine≥10 white blood cells/HPF).1) Of those laboratory criteria, in this study the CRP level, platelets after 7 days, and pyuria were significantly higher in infants than in children. Therefore, those tests should be done and more closely observed than other laboratory data.
There is no specific diagnostic laboratory test for KD, although new diagnostic markers have been studied. In an effort to find these new diagnostic tools, brain natriuretic peptide (BNP) or NT-proBNP has become more important in identifying KD. BNP is a cardiac hormone that is secreted mainly by the left ventricle.22) NT-proBNP is a 76 amino acid N-terminal inactive protein fragment that is cleaved from proBNP to release BNP.23) KD can cause inflammation in the cardiovascular system, including the pericardium, myocardium, endocardium, valves, and coronary arteries.1) That is why BNP and NT-proBNP are used in identifying KD. In this study, the serum level of NT-proBNP was significantly higher in the infants than the children with KD. Furthermore, infantile KD patients showed a significantly higher level of NT-proBNP than those with other febrile diseases. Therefore, it is considered as a useful marker for the early diagnosis of KD in infants. In this study, the optimal range of cut-off value, which differentiates KD from other febrile diseases, was 700 pg/mL, which means febrile patients with above 700 pg/mL of NT-proBNP should be highly suspected of KD. However, even those who have an NT-proBNP below 700 pg/mL should not be excluded from the possibility of KD if they have other features or laboratory findings for patients suspected of KD. Because there have been few studies about the optimal cut-off value of NT-proBNP, more research is needed to elucidate it.
To the best of our knowledge, there has been little study about the comparison of infantile KD patients versus infantile patients with other acute febrile diseases. In this study, there were several significant findings to help differentiate KD from other acute febrile disease. First of all, BCGitis was the striking feature that was only presented in KD patients. Although the WBC counts was not meaningful, the serum levels of ESR and CRP were significantly different, even in the comparison of patients with long fever duration (≥5 days). Furthermore, the NT-proBNP levels was the most prominent marker among laboratory data; its sensitivity and specificity was markedly better than the other laboratory markers.
In conclusion, because the incomplete form of KD is much more common in infants who are more susceptible for development of coronary artery abnormalities, the diagnosis of infantile KD can be difficult. The BCGitis is a unique and common phenomenon in infants with KD, and it should be considered as a significant finding for KD, as well as the classic criteria. The NT-proBNP is also a good predictor of KD, and it is helpful to diagnose and identify KD in febrile infants. These additional features may facilitate an early diagnosis of infantile KD, and help to start treatment early in infants with the incomplete form of KD.
Figures and Tables
Table 1
Comparison of demographic data and clinical features in the infants and the children with Kawasaki disease

Table 2
Comparison of laboratory data in the infants and the children with Kawasaki disease

Table 3
The frequency of coronary artery dilation and retreatment of IVIG in the infants and the children with Kawasaki disease

| Total | Infants | Children | p | |
|---|---|---|---|---|
| CAD | 7 (10.9) | 3 (15.0) | 4 (9.1) | 0.483 |
| IVIG≥2 times | 5 (7.8) | 4 (20.0) | 1 (2.3) | 0.014 |
Table 4
Comparison of the laboratory data in the infants with KD and the infants with other acute febrile diseases

Data are expressed as mean±standard deviation (median). KD: Kawasaki disease, AFD: acute febrile diseases, BCGitis: Erythema and induration at the Bacille Calmette-Guérin inoculation site, WBC: white blood cells counts, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, NT-proBNP: N-terminal pro-brain natriuretic peptide
References
1. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110:2747–2771.
2. Honkanen VE, McCrindle BW, Laxer RM, Feldman BM, Schneider R, Silverman ED. Clinical relevance of the risk factors for coronary artery inflammation in Kawasaki disease. Pediatr Cardiol. 2003; 24:122–126.
3. Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009; 124:e410–e415.
4. Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, and Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001; 103:335–336.
5. Ayusawa M, Sonobe T, Uemura S, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. 2005; 47:232–234.
6. Seo JH, Yu JJ, Ko HK, Choi HS, Kim YH, Ko JK. Diagnosis of incomplete kawasaki disease in infants based on an inflammation at the bacille calmette-guérin inoculation site. Korean Circ J. 2012; 42:823–829.
7. Yoshimura K, Kimata T, Mine K, Uchiyama T, Tsuji S, Kaneko K. N-terminal pro-brain natriuretic peptide and risk of coronary artery lesions and resistance to intravenous immunoglobulin in Kawasaki disease. J Pediatr. 2013; 162:1205–1209.
8. Olivieri L, Arling B, Friberg M, Sable C. Coronary artery Z score regression equations and calculators derived from a large heterogeneous population of children undergoing echocardiography. J Am Soc Echocardiogr. 2009; 22:159–164.
9. Japan Kawasaki Disease Research Committee. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo: Ministry of Health and Welfare;1984.
10. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967; 16:178–222.
11. Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996; 94:1379–1385.
12. Chang FY, Hwang B, Chen SJ, Lee PC, Meng CC, Lu JH. Characteristics of Kawasaki disease in infants younger than six months of age. Pediatr Infect Dis J. 2006; 25:241–244.
13. Chuang CH, Hsiao MH, Chiu CH, Huang YC, Lin TY. Kawasaki disease in infants three months of age or younger. J Microbiol Immunol Infect. 2006; 39:387–391.
14. Hsieh YC, Wu MH, Wang JK, Lee PI, Lee CY, Huang LM. Clinical features of atypical Kawasaki disease. J Microbiol Immunol Infect. 2002; 35:57–60.
15. Perrin L, Letierce A, Guitton C, Tran TA, Lambert V, Koné-Paut I. Comparative study of complete versus incomplete Kawasaki disease in 59 pediatric patients. Joint Bone Spine. 2009; 76:481–485.
16. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993; 87:1776–1780.
17. Sinha R, Balakumar T. BCG reactivation: a useful diagnostic tool even for incomplete Kawasaki disease. Arch Dis Child. 2005; 90:891.
18. Chalmers D, Corban JG, Moore PP. BCG site inflammation: a useful diagnostic sign in incomplete Kawasaki disease. J Paediatr Child Health. 2008; 44:525–526.
19. Uehara R, Igarashi H, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease patients with redness or crust formation at the Bacille Calmette-Guérin inoculation site. Pediatr Infect Dis J. 2010; 29:430–433.
20. Lai CC, Lee PC, Wang CC, Hwang BT, Meng CC, Tsai MC. Reaction at the bacillus Calmette--Guérin inoculation site in patients with Kawasaki disease. Pediatr Neonatol. 2013; 54:43–48.
21. Plantin P, Blayo M, Dupré D, Schoenlaub P. BCG reactivation: a rare but specific sign of Kawasaki disease. Presse Med. 1998; 27:716.
22. Mukoyama M, Nakao K, Saito Y, et al. Human brain natriuretic peptide, a novel cardiac hormone. Lancet. 1990; 335:801–802.
23. Hunt PJ, Yandle TG, Nicholls MG, Richards AM, Espiner EA. The amino-terminal portion of pro-brain natriuretic peptide (Pro-BNP) circulates in human plasma. Biochem Biophys Res Commun. 1995; 214:1175–1183.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download