Abstract
Heart failure is a complex pathophysiological syndrome that can occur in children from a variety of diseases, including cardiomyopathies, myocarditis, and congenital heart disease. The condition is associated with a high rate of morbidity and mortality and places a significant burden on families of affected children and to society as a whole. Current medical therapy is taken largely from the management of heart failure in adults, though clear survival benefit of these medications are lacking. Ventricular assist devices (VADs) have taken an increasingly important role in the management of advanced heart failure in children. The predominant role of these devices has been as a bridge to heart transplantation, and excellent results are currently achieved for most children with cardiomyopathies. There is an ongoing investigation to improve outcomes in high-risk populations, such as small infants and those with complex congenital heart disease, including patients with functionally univentricular hearts. Additionally, there is an active investigation and interest in expansion of VADs beyond the predominant utilization as a bridge to a heart transplant into ventricular recovery, device explant without a heart transplantation (bridge to recovery), and placement of devices without the expectation of recovery or transplantation (destination therapy).
Heart failure has been defined as a clinical and pathophysiological syndrome that results from ventricular dysfunction, volume or pressure overload, either alone or in combination.1) In children, it leads to characteristic signs and symptoms such as poor growth, feeding difficulties, respiratory distress, exercise intolerance, and fatigue.1)Heart failure is one of the most important pathophysiological syndromes in industrialized nations in terms of overall mortality, morbidity, and cost. The Korean Heart Failure Registry described 3200 heart failure hospitalizations among twenty-four hospitals from 2004 to 2009.2) Survival four years after hospital admission was only 70%. In the United States, it is currently estimated that greater than five million adults have heart failure with projections reaching greater than eight million by 2030.3) One out of nine death certificates mention heart failure, and the mortality at five-years after the diagnosis of heart failure remains at approximately 50%.4) The costs associated with disease is staggering, with estimates that the total annual cost of heart failure in the United States will be nearly $70 billion by 2030.5) Similar data exist from other European and Asian countries.2)6)7)8)
Though the etiology of heart failure often differs from that of adults, children are not immune from the burden of heart failure. Massin et al.9) reviewed all cardiac admissions at a tertiary pediatric center in Belgium and found that heart failure occurred in 10% of patients, ranging from 6% of patients with congenital heart disease and 80% for cardiomyopathies. Hospital mortality ranged from 4.7% for children with congenital heart disease to 25.0% for cardiomyopathies. In the United States, there are roughly 14000 hospitalizations annually which approximates eighteen admissions per 100000 children.10) This ranks heart failure among the more common serious acute onset conditions of childhood.11) The majority of these children have some form of congenital heart disease, with about 15% having a cardiomyopathy or myocarditis. The disease carries a substantially increased risk of death with an over twentyfold increased risk of hospital mortality compared to pediatric patients without heart failure. Heart failure is a morbid condition in hospitalized patients with respiratory failure, renal failure, and sepsis occurring in a substantial minority of patients. Moreover, these morbidities are associated with a significant increase in the risk of death (Fig. 1). Additionally, these hospitalizations are lengthy, with the average length of stay being nearly twenty days in 2006, placing a substantial burden on families and society. The median hospital charges per admission were over $70000 in 2009 and this amount does not taken into account of the total cost of care beyond the hospitalization or other costs including missed work by parents.12)
The number of children with chronic heart failure is difficult to ascertain, in part due to the diverse nature of diseases that can lead to heart failure. There are many patients at risk for heart failure from a number of disorders, including congenital heart disease, myocarditis, cardiomyopathy, metabolic disorders, and effects of medications (e.g., anthracyclines). However, not all patients at risk of heart failure will develop heart failure. The incidence of dilated cardiomyopathy form large population-based studies in the United States and Australia range from 0.57 to 0.73 per 100000 children per year.13)14) Not all of these patients will have heart failure at the time of diagnosis, and this number likely underestimates the true incidence as there are likely those with the disease that have not yet been identified. In a prospective study from the United Kingdom, the annual incidence of new-onset heart failure from heart muscle disease was found to be 0.87 per 100000 children who were less sixteen years old.15) Only 66% of these patients were alive or had no transplant one year later.15) This high risk of death or transplant has been confirmed in multiple single center and multi-center reports, with the five-year transplant free survival ranging from 50 to 65% (Fig. 2).16)17)18) The risk for death and transplant is greatest after the initial diagnosis. After one-year, the risk drops significantly with reported events in the range of 1% per year.19) However, not all patients deteriorate, and a significant minority of patients will have a meaningful recovery of ventricular function.20)21) Clearly, further study is needed to understand the worldwide incidence and prevalence of heart failure to understand the true burden of disease and the impact on society.
For patients with new onset heart failure, the diagnosis can be made form a combination of history, exam findings, laboratory studies, and imaging. Children often present with respiratory and gastrointestinal symptoms, and the severity can range from mild to cardiogenic shock.22) A family history of cardiomyopathy and sudden death may be important clues to a genetic etiology. In addition, to standard laboratory assessment, natriuretic peptides are usually significantly elevated and their trend over time can have important predictive value.23)24) Children that do not have a decrease in their natriuretic peptide levels over time are at increased risk of death, requiring a ventricular assist device (VAD) or needing a heart transplant.
Imaging studies are critical to making the diagnosis of heart failure. Chest X-rays are useful for determination of cardiomegaly and pulmonary edema, though specific cardiac imaging with echocardiogram and/or cardiac MRI is needed. For most forms of cardiomyopathy, the ventricular function will be depressed, though patients with restrictive or hypertrophic cardiomyopathy can have heart failure with preserved ventricular function. While echocardiogram remains the most frequently utilized cardiac imaging modality, cardiac MRI is increasingly becoming utilized. In addition to demonstrating structural abnormalities, ventricular size, and ventricular function, assessments of myocardial inflammation and myocardial scar/fibrosis can be performed.25) These assessments can be useful in determining the etiology of heart muscle disease. Additionally, certain findings, such as diffuse fibrosis as evidenced by T1 mapping, may offer some prognostic information.26) Genetic testing and counseling has also become increasingly important in the diagnosis and management of patients with cardiomyopathy.27)
Unlike heart failure therapy in adults, there are few evidencebased data to guide heart failure management in children. There are multiple large prospective multicenter randomized controlled trials in adult heart failure patients with reduced ejection fraction demonstrating the survival advantage of beta-blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists.28)29)30)31)32) However, many drugs shown to be beneficial in the treatment of heart failure in adults have not been proven to be effective in children.18)33)34)35)36)37)
The reasons for the differences in pediatric and adult studies are not entirely clear and are likely multifactorial.38) It is difficult and expensive to perform high quality prospective studies in pediatric patients with sufficient follow-up and clinically meaningful outcomes. The etiologies of heart failure and comorbid conditions in children and adult are different. As such, the molecular mechanisms, gene expression, microRNA expression, and response to medications may be different in children and adults.39) 40)41)42) Moreover, there are limited data on the pharmacokinetics and pharmacodynamics of heart failure medications in children, and this limited amount of information may lead to inadequate dosing. For example, pharmacokinetics studies of carvedilol suggested that infants and young children may require an increase in the dosing and frequency than was used in the prospective carvedilol trial to achieve a similar serum level to that found to be beneficial in adults.34)43) It should be mentioned, however, that, even though ACEIs and beta-blockers have not clearly been demonstrated to be beneficial for the overall pediatric heart failure population, some etiologies have been shown to respond well to these medications. Kwon et al.44) reported on twenty-three patients with Duchenne and Becker muscular dystrophy from Seoul National University Children's Hospital who were treated with either enalapril or carvediolol. There was an overall improvement in fractional shortening. Additionally, though there were no randomized controlled trials, several other studies have demonstrated a favorable response to these medications in patients with muscular dystrophy.45)46)47)48) Despite convincing trials of the benefits of these medications for most patients with heart failure with reduced ejection fraction, they are widely utilized among pediatric heart failure specialists and recommended by consensus guidelines.1)49)
Likewise, there are few data to provide evidenced-based recommendations for the management of acute heart failure in children. In following the guidelines for heart failure in adults, diuretics and vasodilators are often utilized for patients with symptoms of congestion and adequate perfusion with inotropes reserved for those with impaired perfusion. There are multiple studies in adult heart failure patients that have found increased side effects and mortality among patients treated with inotropes compared to those not treated with inotropes.50)51)52)53) 54) Whether this conclusion is also true among children with heart failure is still unknown. What is known is that inotropes seem to be used in the majority of patients with cardiomyopathy and heart failure admitted to the intensive care unit, and limited data would suggest that the use of these medications are associated with increased mortality.52)54) Further study, however, is needed to determine if it is the disease severity versus the use of inotropes that is associated with adverse outcomes. There are data that suggest the disease severity is greater in children versus adults that are hospitalized with heart failure.55)
It is arguable that the greatest advance in pediatric heart failure over the last decade has been the increased utilization and success of VADs for patients with end-stage heart failure.56)57) Until relatively recently, the only modality available for small children with profound and refractory hear failure was extracorporeal membrane oxygenation (ECMO). This modality is not ideal for long-term support for heart failure and is one of the strongest risk factors for death while waiting for heart transplantation and for death after transplantation.58)59) The Berlin Heart Excor (Berlin Heart Inc., Woodlands, TX, USA) VAD was first used in the early 1990's in Germany (Fig. 3).60) It was subsequently spread beyond Europe to North America. By the early 2000's, centers in the United States and Canada had gained ample experience with the device and reported good results with a high success rate as a bridge to heart transplantation.56)61) These results led to a prospective multi-center evaluation of the device that compared the VAD to a retrospective cohort of patients treated with ECMO.62) The Excor VAD performed well in the trial with 88-92% patients surviving to heart transplantation or weaning from the device with a favorable neurological outcome (Fig. 4). This result was a significant improvement over ECMO, where there were no patients alive on ECMO after thirty days of support.
While the use of the Excor VAD, in many ways, revolutionized pediatric mechanical circulatory support, there still exists a relatively high adverse side-effect of the device, including a nearly 30% stroke rate.62) Paracorporeal pneumatic pulsatile pumps, like the Excor VAD, have been largely replaced, in adults and older children, by intracorporeal continuous flow VADs, such as the HeartMate II (Thoratec Corp., Pleasanton, CA, USA) and HeartWare HVAD (HeartWare Inc., Framingham, MA, USA) devices.63) These devices in adults have a significantly improved side-effect profile and have allowed many patients to be supported for years with a good quality of life. Limited data in pediatric patients also support the effectiveness of these devices with a favorable side-effect profile.64)65)66)
While many pediatric patients can be well supported with the currently available VADs, there remains certain populations that are still challenging. Small infants with cardiomyopathies still have high adverse events and mortality rate. The National Institutes of Health/National Heart, Lung, and Blood Institute sponsored Pumps for Kids, Infants, and Neonates (PumpKIN) program plans to initiate a trial of the Infant Jarvik VAD in 2015 (Fig. 5).67) Likewise, patients with complex congenital heart disease, including circulatory failure after the Fontan operation, often fare poorly on VADs. As circulatory failure is complex in this population, including the relatively high subset of patients with circulatory failure with relatively preserved ventricular function, a systemic VAD is often inadequate. Limited reports of 'biventricular' support with two VADs or with a total artificial heart suggest patients can be successfully supported to heart transplantation, though further study is needed on the optimal method of support for this population.68)69)
Historically, most of the VAD support in children has been as a bridge to heart transplantation. As the supply of donor organs and organ utilization remain limited in most parts of the world, including the United States and Asia, there are more people with endstage heart failure than available organs for heart transplantation.70)71) Currently in the United States, approximately 40% of VADs in adults are placed as destination therapy, with placement of the VAD having no expectation of a heart transplantation.63) The use of destination therapy increased dramatically after the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure trial that demonstrated improved survival and quality of life with VAD support in patients with end-stage heart failure that were not eligible for a heart transplantation compared to optimal medical therapy.72) While these indications are still relatively rare in pediatric patients, it has been utilized in certain populations, such as those with Duchenne muscular dystrophy.73) It is likely that use of VADs for patients not eligible for a heart transplantation will continue to grow in the coming years.
Some patients on VADs have meaningful recovery of ventricular function while on support, and some patients have undergone VAD explant and have remained free from heart failure.74) There is mechanical unloading of the ventricle while on VAD support, leading to multiple potential advantageous processes, including decreased wall stress, improved cytoskeletal architecture, improvement neurohormonal profile, and decreased myocardial hypertrophy and death.75)76) Some children have been explanted from VAD due to functional recovery, though most have been in the setting of acute heart failure from myocarditis, graft failure in transplant recipients, or post-cardiotomy heart failure.77)78)79) Given the limitations of heart transplantation, understanding and expansion of VAD utilization as a mechanism for myocardial recovery is of paramount importance in pediatric heart failure.
Heart failure is a common and severe condition that occurs in many children with congenital and acquired heart disease. Though evidence is lacking for much of the medial therapy, many patients can be successfully managed on medications and remain symptom free for a prolonged period of time. A significant proportion of these patients, however, will progress to advanced heart failure, and many will require mechanical circulatory support. There have been tremendous advancements in VAD therapy over the last decade; however, current strategies and devices are often inadequate for small children and those with complex congenital heart disease. Ongoing work is focused on these populations and to expanding the therapy beyond a bridge to heart transplantation into destination therapy and as bridge to recovery.
Figures and Tables
Fig. 1
Hospital mortality of children with heart failure related hospitalizations. *Significantly increased hospital mortality (p<0.05). HTN: hypertension, CVD: cerebrovascular disease, ECMO: extra corporeal membrane oxygenation, VAD: ventricular assist device. Adopted from Rossano JW, et al. with permission from the publisher.10)
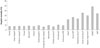
Fig. 2
Freedom from death or transplantation for patients with pure dilated cardiomyopathy. Retrospective cohort diagnosed 1990-1995; Prospective cohort diagnosed 1996-2002. Adopted from Towbin JA, et al. with permission from the publisher.17)

Fig. 3
The Berlin Heart Excor (Berlin Heart Inc.) ventricular assist device. Reproduced with permission from the company. A: application in failing heart. B: magnification of device.
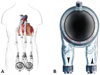
Fig. 4
Outcomes of the Prospective Trial of the Berlin Excor (Berlin Heart Inc.) ventricular assist device to a historical cohort of extracorporeal membrane oxygenation (ECMO) patients. A: cohort 1 included patients with a body surface area <0.7 m2. B: cohort 2 included patients with body surface area 0.7 to <1.5 m2. Adopted from Fraser CD Jr, et al. with permission from the publisher.62)

Fig. 5
The Infant-Size Pediatric Jarvik 200, part of the national heart, lung, and blood institute pediatric circulatory support program. Adopted from Baldwin JT, et al. with permission from the publisher.67)
References
1. Kirk R, Dipchand AI, Rosenthal DN, et al. The International Society of Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: executive summary. J Heart Lung Transplant. 2014; 33:888–909.
2. Choi DJ, Han S, Jeon ES, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean heart failure registry. Korean Circ J. 2011; 41:363–371.
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014; 129:e28–292.
4. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004; 292:344–350.
5. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013; 6:606–619.
6. Tavazzi L, Senni M, Metra M, et al. Multicenter prospective observational study on acute and chronic heart failure: one-year follow-up results of IN-HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013; 6:473–481.
7. Shiba N, Shimokawa H. Chronic heart failure in Japan: implications of the CHART studies. Vasc Health Risk Manag. 2008; 4:103–113.
8. McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart. 2000; 83:596–602.
9. Massin MM, Astadicko I, Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol. 2008; 31:388–391.
10. Rossano JW, Kim JJ, Decker JA, et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail. 2012; 18:459–470.
11. Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. 2013; 14:686–693.
12. Nandi D, Lin KY, O'Connor MJ, et al. Hospital charges for pediatric heart failure related hospitalization in the United States from 200-2009. J Am Coll Cardiol. 2014; 33:S84. Abstract.
13. Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003; 348:1647–1655.
14. Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003; 348:1639–1646.
15. Andrews RE, Fenton MJ, Ridout DA, Burch M;. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United Kingdom and Ireland. Circulation. 2008; 117:79–84.
16. Daubeney PE, Nugent AW, Chondros P, et al. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 2006; 114:2671–2678.
17. Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006; 296:1867–1876.
18. Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010; 55:1377–1384.
19. Alexander PM, Daubeney PE, Nugent AW, et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: results from a national population-based study of childhood cardiomyopathy. Circulation. 2013; 128:2039–2046.
20. O'Sullivan JJ, Roche SL, Crossland DS, Chaudhari MP, Kirk RC, Asif H. Recovery of heart function in children with acute severe heart failure. Transplantation. 2008; 85:975–979.
21. Everitt MD, Sleeper LA, Lu M, et al. Recovery of echocardiographic function in children with idiopathic dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. J Am Coll Cardiol. 2014; 63:1405–1413.
22. Macicek SM, Macias CG, Jefferies JL, Kim JJ, Price JF. Acute heart failure syndromes in the pediatric emergency department. Pediatrics. 2009; 124:e898–e904.
23. Wong DT, George K, Wilson J, et al. Effectiveness of serial increases in amino-terminal pro-B-type natriuretic peptide levels to indicate the need for mechanical circulatory support in children with acute decompensated heart failure. Am J Cardiol. 2011; 107:573–578.
24. Price JF, Thomas AK, Grenier M, et al. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation. 2006; 114:1063–1069.
25. Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013; 309:896–908.
26. Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012; 5:726–733.
27. Hershberger RE, Lindenfeld J, Mestroni L, et al. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J Card Fail. 2009; 15:83–97.
28. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991; 325:293–302.
29. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001; 344:1651–1658.
30. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000; 355:1582–1587.
31. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999; 341:709–717.
32. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003; 362:7–13.
33. Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010; 122:333–340.
34. Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007; 298:1171–1179.
35. Dore A, Houde C, Chan KL, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005; 112:2411–2416.
36. Hechter SJ, Fredriksen PM, Liu P, et al. Angiotensin-converting enzyme inhibitors in adults after the Mustard procedure. Am J Cardiol. 2001; 87:660–663. A11.
37. van der Bom T, Winter MM, Bouma BJ, et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013; 127:322–330.
38. Rossano JW, Shaddy RE. Update on pharmacological heart failure therapies in children: do adult medications work in children and if not, why not? Circulation. 2014; 129:607–612.
39. Stauffer BL, Russell G, Nunley K, Miyamoto SD, Sucharov CC. miRNA expression in pediatric failing human heart. J Mol Cell Cardiol. 2013; 57:43–46.
40. Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2014; 35:33–41.
41. Bernstein D, Fajardo G, Zhao M. The role of β-adrenergic receptors in heart failure: differential regulation of cardiotoxicity and cardioprotection. Prog Pediatr Cardiol. 2011; 31:35–38.
42. Reddy S, Zhao M, Hu DQ, et al. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics. 2012; 44:562–575.
43. Albers S, Meibohm B, Mir TS, Läer S. Population pharmacokinetics and dose simulation of carvedilol in paediatric patients with congestive heart failure. Br J Clin Pharmacol. 2008; 65:511–522.
44. Kwon HW, Kwon BS, Kim GB, et al. The effect of enalapril and carvedilol on left ventricular dysfunction in middle childhood and adolescent patients with muscular dystrophy. Korean Circ J. 2012; 42:184–191.
45. Jefferies JL, Eidem BW, Belmont JW, et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation. 2005; 112:2799–2804.
46. Duboc D, Meune C, Pierre B, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years' follow-up. Am Heart J. 2007; 154:596–602.
47. Viollet L, Thrush PT, Flanigan KM, Mendell JR, Allen HD. Effects of angiotensin-converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in Duchenne muscular dystrophy. Am J Cardiol. 2012; 110:98–102.
48. Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Bécane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005; 45:855–857.
49. Kantor PF, Lougheed J, Dancea A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013; 29:1535–1552.
50. Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991; 325:1468–1475.
51. Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005; 46:57–64.
52. Price JF, Mott AR, Dickerson HA, et al. Worsening renal function in children hospitalized with decompensated heart failure: evidence for a pediatric cardiorenal syndrome? Pediatr Crit Care Med. 2008; 9:279–284.
53. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002; 287:1541–1547.
54. Shamszad P, Hall M, Rossano JW, et al. Characteristics and outcomes of heart failure-related intensive care unit admissions in children with cardiomyopathy. J Card Fail. 2013; 19:672–677.
55. Wittlieb-Weber CA, Lin KY, Zaoutis TE, et al. Pediatric versus adult cardiomyopathy and heart failure related hospitlaizations: a valuebased analysis. Circulation. 2014; 128:A11498. Abstract.
56. Morales DL, Almond CS, Jaquiss RD, et al. Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant. 2011; 30:1–8.
57. Rossano JW, Mott AR, Mohamad Z, et al. Decreasing mortality of ventricular assist devices at children's hospitals from 2000-2010: improvement at a cost (abstract). Circulation. 2012; 126:A11553.
58. Almond CS, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009; 119:717–727.
59. Benden C, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Lung and Heart-Lung Transplantation Report--2013; focus theme: age. J Heart Lung Transplant. 2013; 32:989–997.
60. Hetzer R, Alexi-Meskishvili V, Weng Y, et al. Mechanical cardiac support in the young with the Berlin Heart EXCOR pulsatile ventricular assist device: 15 years' experience. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006; 99–108.
61. Imamura M, Dossey AM, Prodhan P, et al. Bridge to cardiac transplant in children: Berlin Heart versus extracorporeal membrane oxygenation. Ann Thorac Surg. 2009; 87:1894–1901. discussion 1901.
62. Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012; 367:532–541.
63. Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013; 32:141–156.
64. Cabrera AG, Sundareswaran KS, Samayoa AX, et al. Outcomes of pediatric patients supported by the HeartMate II left ventricular assist device in the United States. J Heart Lung Transplant. 2013; 32:1107–1113.
65. Miera O, Potapov EV, Redlin M, et al. First experiences with the Heart-Ware ventricular assist system in children. Ann Thorac Surg. 2011; 91:1256–1260.
66. Padalino MA, Bottio T, Tarzia V, et al. HeartWare ventricular assist device as bridge to transplant in children and adolescents. Artif Organs. 2014; 38:418–422.
67. Baldwin JT, Borovetz HS, Duncan BW, Gartner MJ, Jarvik RK, Weiss WJ. The national heart, lung, and blood institute pediatric circulatory support program: a summary of the 5-year experience. Circulation. 2011; 123:1233–1240.
68. Rossano JW, Goldberg DJ, Fuller S, Ravishankar C, Montenegro LM, Gaynor JW. Successful use of the total artificial heart in the failing Fontan circulation. Ann Thorac Surg. 2014; 97:1438–1440.
69. VanderPluym CJ, Rebeyka IM, Ross DB, Buchholz H. The use of ventricular assist devices in pediatric patients with univentricular hearts. J Thorac Cardiovasc Surg. 2011; 141:588–590.
70. Rossano JW, Lin KY, Paridon SM, et al. Pediatric heart transplantation from donors with depressed ventricular function: an analysis of the United Network of Organ Sharing Database. Circ Heart Fail. 2013; 6:1223–1229.
71. Cha MJ, Lee HY, Cho HJ, Hwang HY, Kim KB, Oh BH. Under-utilization of donor hearts in the initial era of the heart transplant program in Korea- review of 13 years' experience from the Korea national registry. Circ J. 2013; 77:2056–2063.
72. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001; 345:1435–1443.
73. Amodeo A, Adorisio R. Left ventricular assist device in Duchenne cardiomyopathy: can we change the natural history of cardiac disease. Int J Cardiol. 2012; 161:e43.
74. Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006; 355:1873–1884.
75. Drakos SG, Kfoury AG, Stehlik J, et al. Bridge to recovery: understanding the disconnect between clinical and biological outcomes. Circulation. 2012; 126:230–241.
76. Vatta M, Stetson SJ, Jimenez S, et al. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J Am Coll Cardiol. 2004; 43:811–817.
77. Wilmot I, Morales DL, Price JF, et al. Effectiveness of mechanical circulatory support in children with acute fulminant and persistent myocarditis. J Card Fail. 2011; 17:487–494.
78. Morales DL, Braud BE, Price JF, et al. Use of mechanical circulatory support in pediatric patients with acute cardiac graft rejection. ASAIO J. 2007; 53:701–705.
79. Irving CA, Crossland DS, Haynes S, Griselli M, Hasan A, Kirk R. Evolving experience with explantation from Berlin Heart EXCOR ventricular assist device support in children. J Heart Lung Transplant. 2014; 33:211–213.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download