Abstract
Acute giant coronary aneurysm after Kawasaki disease (KD) is a catastrophic complication that can be fatal and very difficult to manage. However, no fixed consensus has been reached for the management of super-giant coronary aneurysms in the acute setting. Here, we report the successful management of young children with super-giant coronary aneurysms after KD. Based on our experience, hemodynamic stabilization to prevent further coronary dilation or rupture and strict anticoagulation to avoid thrombus formation are mandatory in the management of this condition.
Kawasaki disease (KD) is a self-limiting systemic inflammatory disease of childhood,1) and the most important issue with this condition is coronary artery complications. The prevalence of coronary artery involvement is approximately 15-25%, but it decreases to 5% after the introduction of intravenous immunoglobulin (IVIG) treatment.2)
A giant coronary artery aneurysm (CAA) has a diameter of >8 mm3)4) and occurs at an incidence of approximately 0.25-2% in patients with KD.5)6) In addition, KD patients with giant CAA show the greatest risk of stenosis and obstruction of the coronary artery, and even myocardial infarction during follow-up. Moreover, several case reports have documented aneurysmal rupture, cardiac tamponade, and death in the acute phase.7-10) Despite the seriousness of giant CAA, no fixed consensus has been achieved in the management of this condition in the acute setting. Here, we report 2 cases of successful management of super-giant CAA in the acute setting.
A 31-month-old girl presenting with a fever was admitted to a nearby hospital. She showed bilateral conjunctival injection, swelling of the hands and feet, enlargement of the cervical lymph nodes, and a polymorphous rash. Because she was considered to have KD, IVIG (2 g/kg) and high-dose aspirin were given to her on the 5th day of illness. Because the fever persisted, she received additional IVIG (2 g/kg) on the 7th day. Intravenous methylprednisolone was infused on the 9th day because of persistent fever. Echocardiography showed a mildly enlarged coronary artery. The fever dropped on the 16th day, and she was discharged with oral methylprednisolone (2 mg/kg∙day) and low-dose aspirin (5 mg/kg∙day).
At the outpatient department on the 25th day of illness, her echocardiography revealed that the right coronary artery (RCA) was severely dilated up to 16 mm in diameter. She was transferred to our hospital. On admission, her vital signs were as follows: blood pressure, 120/60 mm Hg; pulse rate, 140 beats/min; respiratory rate, 24 breaths/min; temperature, 37.6℃. Her great toe tip and sole were desquamated. Her platelet count was 648000/uL; erythrocyte sedimentation rate, 72 mm/h; and serum C-reactive protein, 0.42 mg/dL. Her serum creatine kinase-MB was normal. However, her serum troponin I level was higher than normal at 0.58 ng/mL. The echocardiography showed giant CAA and thrombus formation in the left anterior descending artery (LAD), RCA, and left circumflex artery (LCX). The maximal diameter of the RCA, LAD, and LCX was 16 mm, 9.9 mm, and 7.5 mm, respectively. The flow through coronary artery was intact (Fig. 1).
She was transferred to the intensive care unit because she was at a high risk of aneurysmal rupture or myocardial infarction. She was intubated and deeply sedated with midazolam, ketamine, and vecuronium, and was also given continuous intravenous esmolol and nicardipine. For preventing further thrombus formation, intravenous heparin was infused continually, and oral methylprednisolone was tapered off on schedule. After stabilization of her heart rate and blood pressure, we added oral atenolol (2 mg/kg∙day) and amlodipine (0.1 mg/kg∙day) to stop the intravenous esmolol and nicardipine and replaced the intravenous heparin with warfarin. On the 46th day of illness, she returned to the general ward. Despite the adequate dose of aspirin and warfarin, echocardiography on the 57th day showed that the thrombi had reappeared in the RCA. Therefore, we added clopidogrel (0.75 mg/day) to her medication.
She underwent cardiac computed tomography (CT), which revealed aneurysmal dilatation of coronary arteries. The maximal diameter of the proximal LAD, LCX, and RCA was 10.8 mm, 7.4 mm, and 18 mm, respectively. She was discharged with oral aspirin, warfarin, clopidogrel, atenolol, and amlodipine on the 68th day. On the 9th month of illness, she underwent cardiac CT and echocardiography. The size of the CAAs had not increased, while the thrombus in the RCA had disappeared (Fig. 2). At the last follow-up visit, which was 10 months after the onset of KD, the patient was doing well without any symptoms.
A 4-year-old boy was admitted to a nearby hospital because of fever. He showed conjunctival injection, enlargement of cervical lymph nodes, and edema and erythema of the hands and feet. Under the diagnosis of KD, he was given 2 g/kg of IVIG and high-dose aspirin. Because his fever did not subside, additional IVIG (2 g/kg) was given on the 6th day. On the 8th day, 1 mg/kg∙day of oral prednisolone was started; the fever subsided 2 days later. He was discharged with oral prednisolone (0.5 mg/kg∙day) on the 11th day, and prednisolone was discontinued on the 29th day.
On the 32nd day of illness, echocardiography at the outpatient department showed multiple giant CAAs. Cardiac CT also revealed a giant, bead-shaped, fusiform aneurysm in multiple coronary arteries; the diameter of the RCA, left main coronary artery (LMCA), and LAD was 22.3 mm, 7.9 mm, and 16.8 mm, respectively. CT scan revealed multiple thrombi in the LCX, RCA, and LAD, as well as multifocal stenosis in the LCX. Oral warfarin, aspirin, and amlodipine were started, and the patient was transferred to our hospital on the 41st day of illness. On admission, he denied any symptoms. His vital signs were as follows: blood pressure, 99/70 mm Hg; pulse rate, 109 beats/min; respiratory rate, 24 breaths/min; temperature, 36.7℃. His fingertips were desquamated.
We added oral carvedilol (0.28 mg/kg∙day) to decrease his blood pressure and heart rate. On the 44th day, repeated cardiac CT was performed to determine whether an emergency operation was necessary; the largest diameter of the proximal RCA, LMCA, and LAD was 23 mm, 7 mm, and 14 mm, respectively. Multiple thrombi were observed in the LAD and RCA, while the thrombus in the proximal LAD lesion had decreased (Fig. 3). Because no significant change had occurred since the previous cardiac CT, we continued the medical treatment. The thrombus in the RCA had nearly disappeared on the 53rd day. On the 67th day of illness, the thrombus in the LAD looked organized, and the diameter of the coronary arteries did not change on follow-up echocardiography. He was discharged with warfarin, aspirin, amlodipine, and carvedilol (1.3 mg/kg∙day).
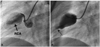
On the 109th day of illness, a large thrombus on the RCA had developed, and cardiac enzyme was elevated at a routine check-up visit after a dental procedure. Before the dental procedure, warfarin was replaced by subcutaneous enoxaparine. He underwent emergent coronary angiography and intracoronary urokinase (4000 IU/kg) infusion (Fig. 4), but there was little effect. We added clopidogrel (1.1 mg/kg∙day) and maintained intravenous heparin for 8 days. He was subsequently discharged without any symptoms and further complications. At the last follow-up visit (9 months after the onset of KD), the patient was doing well without any symptoms.
We reported here 2 cases of super-giant CAA after KD. In both cases, the coronary arteries were severely dilated, and thrombus had developed within a month after the onset of KD, which needed strict hemodynamic stabilization to prevent further dilatation and even aneurysmal rupture. Imai et al.8) demonstrated that rapidly growing CAAs that achieve a diameter of more than 10 mm should be termed super-giant CAAs. Moreover, we had to maintain anticoagulation using heparin, followed by warfarin, to eliminate multiple thrombi and to avoid further thrombus formation. However, a thrombus developed again several months in the CAA of both patients. In the first case, we added another antiplatelet drug, clopidogrel, to the previous medication within 2 months of the illness. In the second case, the patient experienced myocardial infarction after a dental procedure and had to receive emergency intracoronary thrombolysis within 4 months of the illness. In both cases, we did not perform intracoronary thrombosuction, since we worried about further thrombus formation during the procedure.
Although no fixed guidelines have been developed for the management of giant CAA in the acute setting, further dilatation and even rupture of the CAA, as well as thrombus formation, should be avoided. Moreover, in the treatment of giant CAA, the following points should be considered. First, the hemodynamic force is important for the size of the aneurysm. Therefore, blood pressure and heart rate should be strictly controlled by beta blockers and other anti-hypertensive medications. We made an effort to prevent blood pressure and heart rate from increasing beyond the 50th of normal age- and sex-matched population and to prevent abrupt increases. Imai et al.8) suggested that sedative drugs, calcium channel blockers, and beta blockers could be helpful in lowering blood pressure and heart rate, and could reduce sheer stress on the dilated coronary arterial wall. Second, giant CAA tends to produce thrombi, which result in occlusion of distal vessels and subsequent myocardial infarction. The pathophysiology of thrombus formation in the aneurysm is considered to involve stagnation of flow and reduction of shear stress in the coronary aneurysm. Reduced shear stress promotes platelet aggregation and coagulation and reduces fibrinolysis.11)12) The combination of warfarin and aspirin is known to have high cardiac-event-free survival in patients with giant CAA caused by KD.4) Furthermore, intracoronary urokinase can be meaningful in the treatment and prevention of myocardial infarction in the acute setting of KD.13)
The role and effect of steroids in coronary involvement of KD remains controversial. Kato et al.14) reported that KD patients treated with corticosteroids may have a high risk of developing CAAs. Recently, however, the RAISE trial showed that primary treatment with prednisolone and IVIG improves coronary artery outcomes in patients with severe KD.15) In a meta-analysis of 9 clinical studies with a total of 1011 patients, the combination of corticosteroid with a conventional regimen of IVIG reduced the risk of coronary abnormalities.16) Thus far, the effects of corticosteroids on CAA development and later vascular remodeling are uncertain. Meanwhile, most patients who died of ruptured CAAs were treated with steroids, indicating steroids may be a trigger of aneurysm rupture.7) Further, steroids are associated with poorer pre-existing CAA regression.17) Therefore, steroids should be used carefully in the presence of CAAs. In the first case, we tapered oral steroid to prevent harmful effects on CAAs.
Finally, the role of thrombus formation in aneurysms is a highly important one. An adequate amount of stable intramural thrombus could prevent aneurismal rupture by reducing wall tension in the acute phase of giant aneurysms. However, further studies are necessary to confirm this potential role.
In conclusion, giant CAA has the risk of aneurismal rupture and thrombosis in the acute phase of KD, which results in sudden death. Therefore, more careful and intensive treatment is necessary for giant CAA in the acute phase after KD. Here, we were able to successfully manage 2 cases of super-giant CAA in the acute phase through stabilizing the hemodynamic status and maintaining anticoagulation.
Figures and Tables
Fig. 1
Case 1, initial echocardiogram. A: the RCA showed marked dilatation (16 mm) with thrombus formation. B: the left coronary artery also had a giant aneurysm containing a thrombus in the LAD (9.9 mm). RCA: right coronary artery, LAD: left anterior descending artery, LCX: left circumflex artery.
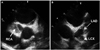
Fig. 2
Case 1, follow-up echocardiogram and computed tomography (CT). A: the RCA showed disappearance of the thrombus; however, a rather giant aneurysm (16.4 mm) was still present 5 months after treatment. B: cardiac CT revealed a tortuous and giant RCA aneurysm (18 mm) without significant thrombus formation. The left coronary artery was also dilated. RCA: right coronary artery, LAD: left anterior descending artery.
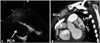
Fig. 3
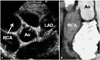
Case 2, initial echocardiogram and computed tomography (CT). A: the RCA showed a marked giant aneurysm (23 mm) with thrombus formation and with a diameter greater than that of the aorta. The left coronary artery also had a giant aneurysm in the LAD (16.8 mm) containing mural thrombus. B: cardiac CT revealed blood stagnation in the RCA. RCA: right coronary artery, LAD: left anterior descending artery, Ao: aorta.
References
1. Burns JC, Kushner HI, Bastian JF, et al. Kawasaki disease: a brief history. Pediatrics. 2000; 106:E27.
2. Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004; 364:533–544.
3. Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996; 94:1379–1385.
4. Suda K, Kudo Y, Higaki T, et al. Multicenter and retrospective case study of warfarin and aspirin combination therapy in patients with giant coronary aneurysms caused by Kawasaki disease. Circ J. 2009; 73:1319–1323.
5. Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012; 22:79–85.
6. McNeal-Davidson A, Fournier A, Scuccimarri R, et al. The fate and observed management of giant coronary artery aneurysms secondary to Kawasaki disease in the Province of Quebec: the complete series since 1976. Pediatr Cardiol. 2013; 34:170–178.
7. Suzuki N, Seguchi M, Kouno C, Inukai K, Kito H, Kobayashi H. Rupture of coronary aneurysm in Kawasaki disease. Pediatr Int. 1999; 41:318–320.
8. Imai Y, Sunagawa K, Ayusawa M, et al. A fatal case of ruptured giant coronary artery aneurysm. Eur J Pediatr. 2006; 165:130–133.
9. Panzer J, De Jaeger A, Suys B. Rupture of giant coronary arterial aneurysm without progressive dilation. Cardiol Young. 2008; 18:189–190.
10. Sunagawa K, Mitsumata M, Ayusawa M, Kusumi Y. Ruptured giant aneurysm of the left anterior descending coronary artery in Kawasaki disease. Pediatr Cardiol. 2008; 29:1115–1119.
11. Kuramochi Y, Ohkubo T, Takechi N, Fukumi D, Uchikoba Y, Ogawa S. Hemodynamic factors of thrombus formation in coronary aneurysms associated with Kawasaki disease. Pediatr Int. 2000; 42:470–475.
12. Ohkubo T, Fukazawa R, Ikegami E, Ogawa S. Reduced shear stress and disturbed flow may lead to coronary aneurysm and thrombus formations. Pediatr Int. 2007; 49:1–7.
13. Kato H, Inoue O, Ichinose E, Akagi T, Sato N. Intracoronary urokinase in Kawasaki disease: treatment and prevention of myocardial infarction. Acta Paediatr Jpn. 1991; 33:27–35.
14. Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979; 63:175–179.
15. Kobayashi T, Saji T, Otani T, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012; 379:1613–1620.
16. Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013; 99:76–82.
17. Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001; 43:211–217.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download