Abstract
Amiodarone is a widely used antiarrhythmic agent. Among its various adverse effects, amiodarone-induced pulmonary toxicity (APT) is the most life threatening complication, which has been described mostly in patients who have been in treatment with high accumulative doses for a long duration of time. However, amiodarone therapy in short-term duration induced APT was rarely reported. We describe a case of a 54-year-old man who is presented with symptoms of APT after a few days of therapy for post-myocardial infarction ventricular tachycardia. For early diagnosis and successful treatment, awareness and high suspicion of this rare type of early onset APT is crucial in patients with amiodarone therapy.
Amiodarone is a benzofuran derivative antiarrhythmic drug that has been commonly used to treat atrial fibrillation as well as supraventricular and ventricular tachycardia. Amiodarone-induced pulmonary toxicity (APT) is one of the various side effects of amiodarone therapy. Because the clinical manifestation of APT resembles pulmonary infection, heart failure, pulmonary thromboembolism and restrictive pulmonary disease,1) diagnosis of APT is difficult and therefore is frequently delayed without strong clinical suspicion. APT has been described mostly in patients who have received a large dose of amiodarone over prolonged periods of time after cardiac surgery or pulmonary angiography. We report here a case of APT after a short course of therapy for post-myocardial infarction ventricular tachycardia. This study will be helpful for increasing awareness of a very early onset of APT.
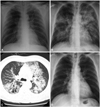
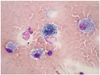
A 54-year-old man was admitted to the hospital with chest pain and ST elevation myocardial infarction. He had no medical history, except for a 60 pack-year smoking history and that he was current smoker. A cardiac catheterization showed total occlusion with Thrombolysis in Myocardial Infarction 0 flow at the proximal left anterior descending artery. He had percutaneous coronary intervention with stenting at the proximal left anterior descending coronary artery. On the night of the catheterization, ventricular tachycardia developed. He was electrically cardioverted, which resulted in sinus rhythm, and amiodarone therapy was started at an initial loading dose of 150 mg intravenously (i.v.), followed by a daily dose of 730 mg i.v. for 2 days. A transthoracic echocardiogram demonstrated anterior and anteroseptal hypokinesis of the left ventricle with an ejection fraction of 35%. After 2 days of amiodarone therapy - a total of 1635 mg of amiodarone - low-grade fever developed with mild dyspnea. Pulse oximetry was 88% in oxygen saturation on room air. Compared with the initial chest X-ray finding, a follow-up chest radiography demonstrated bilateral diffuse infiltration (Fig. 1A and B). Blood tests showed a leukocyte count of 15800/mm3 and a serum creatinine value of 1.2 mg/dL. Pneumonia was suspected and intravenous therapy with piperacillin, tazobactam and levofloxacin was started. The results of blood and sputum culture were negative. Gram's stain and a smear for tuberculosis organisms were also negative, as were serologic tests for Legionella and Chlamydia trachomatis. Mycoplasma pneumoniae polymerase chain reaction tests were also negative. Values for antinuclear antibody, double-stranded deoxyribonucleic acid antibodies, rheumatoid factor, as well as C'3 and C'4 were all within normal limits. Pulmonary edema was also suspected and intravenous diuretics therapy (Lasix 100 mg/day for 3 days) was started; however, no interval change was noted on chest radiography after 3 kg of weight loss. A high resolution computed tomography scan of the chest showed bilateral diffuse consolidation, ground glass opacity and underlying emphysema (Fig. 1C). Owing to a high suspicion of amiodarone pulmonary toxicity (APT), amiodarone was discontinued after a total dose of 4035 mg over more than 8 days. Steroid therapy was not started due to mild respiratory symptoms. Pulmonary function tests showed mild obstructive impairment and carbon monoxide diffusing capacity (DLCO) was checked as 16.1 L (79%). As the clinical status was stabilized, bronchoscopy was performed. Bronchoalveolar lavage revealed a cell count of 200 cells/µL with prominent eosinophilia (8%) and foamy macrophages (Fig. 2). No bacteria or fungi were identified via microscopic examination or culture of the lavage fluid. Ten days after amiodarone was discontinued, the patient was discharged with mild dyspnea. Three months later, APT appeared to be reversible with the resolution of chest radiography findings (Fig. 1D) and recovery of symptoms.
Amiodarone is a highly effective antiarrhythmic that is used to treat atrial fibrillation due to its high conversion rate, rapid onset of action and minimal myocardial depression. However, amiodarone therapy has been associated with various side effects, including pulmonary toxicity, which may occur in up to 5-10% of treated patients.1) APT can present as several manifestations: chronic interstitial pneumonitis, organizing pneumonia, acute respiratory distress syndrome and solitary pulmonary mass.2) The mechanisms of amiodarone-induced pulmonary injury are incompletely understood. Two major hypotheses include direct cytotoxicity and a hypersensitivity reaction.3)
Risk factors for APT include a daily dose greater than 400 mg/day, a duration of therapy exceeding two months, increased patient age, preexisting lung disease, undergoing operation and pulmonary angiography.4) In the present case, the patient usually had no respiratory symptoms; however, a pulmonary function test and high resolution chest computed tomography showed a possibility of chronic lung disease. Additionally, cardiac catheterization was performed. Other cases of APT occurring within a week after cardiac catheterization exist.5)6) Thus, these could be the risk factors for APT. However, in our case, APT occurred only 2 days after 1635 mg of amiodarone. To our knowledge, this represents the most rapidly developed APT.
Amiodarone-induced pulmonary toxicity is a diagnosis of exclusion. Due to its non-specific nature and its ability to mimic other disease processes, such as congestive heart failure, acute pulmonary respiratory syndrome and pneumonia, the diagnosis of APT may be delayed or missed. The presence of foamy macrophages is suggestive of APT, but is not diagnostic. Increased lung attenuation on computed tomography, increased gallium uptake and abnormal pulmonary function tests are nonspecific. A reduction in DLCO of 15% is a strong indicator for the diagnosis of pulmonary toxicity due to amiodarone, with a sensitivity of 68-100% and a specificity of 69-95%.7) Lung biopsy may be helpful in excluding alternative etiologies and may be helpful in identifying the pathologic type of disease (e.g., chronic interstitial pneumonitis); yet, this procedure is also nonspecific for amiodarone-induced disease. In the present case, initial treatment included antibiotics and diuretics because pneumonia and pulmonary edema were suspected. Despite these treatments, symptoms and a chest X-ray were not improved until amiodarone was discontinued. Additionally, foamy macrophages were present in the bronchoalveolar lavage. Thus, our diagnosis was convincing.
Discontinuation of amiodarone is the primary therapy for amiodarone toxicity. Additionally, for patients with symptomatic pulmonary toxicity, systemic steroids may be valuable.7) Due to the long elimination half-life (approximately 45 days) of amiodarone, pulmonary toxicity may initially progress despite the drug discontinuation and may recur upon glucocorticoid withdrawal. In the present case, steroid therapy was not carried out due to mild respiratory symptoms.
Very early onset of APT appears to be unusual without surgery. The present case shows that APT can occur at anytime during therapy. As usage of amiodarone increases, one must be aware of a possible APT, even after a short course of amiodarone therapy.
Figures and Tables
Fig. 1
A: the initial chest X-ray study shows no active lung lesion. B: the follow-up chest X-ray study showed bilateral alveolar and interstitial infiltration. C: the high resolution computed tomography scan of the lung shows bilateral patchy infiltrations with ground glass opacity. D: three months after discharge, the chest X-ray study shows resolution of bilateral infiltration.
References
1. Dusman RE, Stanton MS, Miles WM, et al. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990; 82:51–59.
2. Martin WJ 2nd, Rosenow EC 3rd. Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part I). Chest. 1988; 93:1067–1075.
3. Martin WJ 2nd, Rosenow EC 3rd. Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part 2). Chest. 1988; 93:1242–1248.
4. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008; 66:82–87.
5. Jang WJ, Chon HR, Jung JS, et al. Amiodarone-induced pulmonary toxicity within a short period of the initiation of amiodarone therapy: a case report. Korean J Crit Care Med. 2011; 26:117–121.
6. Tanawuttiwat T, Harindhanavudhi T, Hanif S, Sahloul MZ. Amiodarone-induced alveolar haemorrhage: a rare complication of a common medication. Heart Lung Circ. 2010; 19:435–437.
7. Papiris SA, Triantafillidou C, Kolilekas L, Markoulaki D, Manali ED. Amiodarone: review of pulmonary effects and toxicity. Drug Saf. 2010; 33:539–558.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download