Introduction
Stroke-like episodes are a component of myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome. The infarct-like lesions in the brain might result from the mutation of mitochondrial deoxyribonucleic acid (DNA) that cause defects in translation of the respiratory chain enzymes.1)
Cardiac involvement occurs frequently with MELAS syndrome but there are no case reports or studies about the related risk of intracardiac thrombus which might have been the cause of the stroke.
Case
A 27-year-old female was admitted to the hospital because of left hemiplegia and aphasia. She was 162 centimeters tall and 30 kilograms in weight. She was born after a normal pregnancy and delivery. There was no family history of neurological diseases. Motor and intellectual development was normally attained during infancy. She was hospitalized for general muscle weakness and gait disturbance when she was 6 years old. A neurologic exam showed decreased muscle tone and strength, and atrophic muscle mass. She had consistent muscle weakness, so a muscle biopsy was performed from the calf muscle when she was 8 years old. The biopsy showed mitochondrial myopathy of the pleoconial type. Her first echocardiography was completed afterwards and showed marked hypertrophy of both ventricles without any regional wall problems. Follow-up procedures were performed in an outpatient clinic once or twice a year.
When she was 24 years old she had sudden syncope. An magnetic resonance imaging (MRI) revealed acute infarction of the left basal ganglia and the left frontal lobe. Two years later, when the patient was 26, she had another stroke and presented with general weakness, aphagia, and dysarthria. An MRI showed old multifocal infarctions at the basal ganglia, thalamus, left pons and left periventricular white matter area. An MR spectroscopy showed a positive lactate peak in both basal ganglia. These clinical and radiological findings suggested brain involvement of MELAS syndrome. Thus, further evaluation was done for MELAS syndrome including blood lactate and genetic analysis.
The plasma lactate level was 21.8 mg/dL (normal range 4.5-19.8 mg/dL). CBC, electrolyte, blood urea nitrogen, and creatinine were in the normal range. Thyroid function was also measured. The free T4 was 1.76 ng/dL (normal range 0.89-1.76 ng/dL) and TSH was 0.04 µIU/mL. Levels of complement component 3 and 4 were 13 mg/dL (normal range 75-145 mg/dL) and 18 mg/dL (normal range 12-72 mg/dL) respectively. Antistreptolysin O antibody was negative, rheumatoid factor was negative, and anti-double stranded DNA was 1.6 (normal range 0-6). Lupus anticoagulants and anti cytoplasmic antibody, which were measured to rule out vasculitis, were normal. A DNA gene sequencing study showed a mutation: m.3303C>T mutation in the mitochondrially encoded tRNA leucine 1 gene, which confirmed the diagnosis of MELAS syndrome. She was treated with supportive care and rehabilitation for a month and was then discharged. Warfarin was used during the hospital stay, but was stopped when she was discharged, because MELAS syndrome causes nonvascular infarct and there is no report about the related risk of thromboembolism.
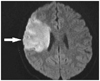
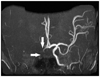
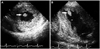
One year later, she presented with another stroke with associated left sided weakness, and was subsequently admitted to the hospital. Her vital signs were stable and there were no specific findings in chest X-rays or electrocardiography. Her MRI revealed infarction in the right middle cerebral territory (Fig. 1). An magnetic resonance angiography showed an occluded right distal internal carotid artery and right middle cerebral artery (Fig. 2). An echocardiography was performed to identify the cardiac origin of the ischemic stroke, and it showed concentrically hypertrophied left ventricle with globally hypokinetic wall motion and ejection fraction of 25%. An intracardiac thrombus attached to the left ventricular apex was noted (Fig. 3). The patient's mental status and general condition improved after she was treated with mannitol and anticoagulation therapy. Rehabilitation and supportive care, including warfarin, were followed and maintained. The patient was discharged after a month.
Discussion
Myopathy, encephalopathy, lactic acidosis and stroke-like episodes is one of the most common mitochondrial disorders inherited maternally, and is frequently associated with mitochondrial DNA mutation encoding transfer ribonucleic acid (RNA), with the most common mutation occurring in the transfer RNA for leucine.2) As a result of the disturbed function of their cells' mitochondria, patients with MELAS syndrome present with various clinical features, including stroke like episodes, cardiomegaly, muscle weakness, headache, vomiting, short stature, dementia, deafness, and endocrine system dysfunction with diabetes mellitus, hypogonadism, and hypoparathyroidism.3) They may also have congestive heart failure with cardiomyopathy, which can progress to conduction defects causing sudden cardiac death.4)
The pathology of stroke-like episodes in MELAS is unclear. So far, it has been assumed that the infarct-like lesions might result from microdeletion or point mutation in the mitochondrial DNA, and that this mutation causes defects in the translation of respiratory chain enzymes. The reduction of oxidative activity results in decreased aerobic respiration causing nonvascular infarcts within the brain parenchyma, and also results in increased metabolic demand, causing anaerobic metabolism activation, parenchyma lactate accumulation and tissue damage.5)6)
Cardiac involvement in MELAS syndrome is reported to be as high as 18-100%.7)8) Cardiac abnormalities manifest as impulse generation or conduction abnormalities, or as abnormalities of the left ventricular myocardium. The most common pathology is non-obstructive concentric hypertrophy, although dilatation is also reported and might be seen as progression of the initial hypertrophic cardiomyopathy.9) In children, cardiomyopathy may actually be the first manifestation of MELAS syndrome. Wolff-Parkinson-White syndrome has also been reported in up to 17% of patients.7)10)
Myopathy, encephalopathy, lactic acidosis and stroke-like episodes syndrome remains largely untreatable, and as such, the morbidity and mortality rates are very high. The prognosis is poor because encephalopathy tends to be severe and progresses quickly to dementia, leading to a state of cachexia. Death results rapidly after cardiac failure, pulmonary embolism, or renal failure. There are only a few case reports suggesting that the use of L-arginine and coenzyme Q10 in addition to vitamin supplementation might be advantageous.
There is no data about the prevalence of intracardiac thrombus causing stroke in the MELAS patients. More studies and reports are needed, but it is clear that more frequent echocardiography can help reduce the chances of more cerebral infarction in a patient with MELAS syndrome. Therefore, cardiac affection in MELAS requires special attention, since patients may be saved from recurrent strokes as well as malignant rhythm abnormalities or heart failure if detected and treated early. They should undergo more careful echocardiography because this may have preventive and prognostic consequences.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download