Abstract
While thoracic endovascular aortic repair is an effective treatment option for descending thoracic aorta pathology, it does have limitations. The main limitation is related to the anatomical difficulties when disease involves the aortic arch. A fenestrated, branched aortic stent graft and hybrid operation has been introduced to overcome this limitation, but it is a custom-made device and is time consuming to manufacture. Furthermore, these devices cannot be used in an emergency setting. We report two patients with massive descending thoracic aortic aneurysm and ruptured aortic dissection very near the aortic arch who underwent a procedure which we named the modified chimney technique. The modified chimney technique can be used as a treatment option in such an emergency situation or as a rescue procedure when aortic pathology is involved near the supra-aortic vessels.
Thoracic endovascular aortic repair (TEVAR) is a less invasive strategy compared to open surgical repair for patients with various types of aortic diseases. According to the recently published meta-analysis of comparative studies on endovascular aortic repair versus open surgical repair for treating descending thoracic aortic disease, the non-randomized evidence showed that TEVAR may reduce early mortality and morbidity when compared with conventional open surgical management for patients who require intervention for descending thoracic aortic disease.1) Despite these advantages, TEVAR has some difficulties. The common problem is the presence of an inadequate short proximal and distal landing zone. To achieve an adequate landing zone and sealing zone, the innominate artery, left carotid artery and left subclavian artery occasionally need to be covered. Modification of the stent graft is needed to overcome these limitations of TEVAR. The use of a fenestrated or branched stent graft, which is able to preserve perfusion of the supra-aortic arch vessels, could be one of the alternative approaches.2) However, a fenestrated or branched stent graft is a custom made device, and is expensive and time consuming to manufacture. Furthermore, these devices cannot be used in an emergency setting.3)4) An alternative approach that can be used in these patients is the "chimney graft" technique to preserve flow to the supra-aortic arch vessels with a short landing zone, which is impossible to repair with a standard stent-graft.5) The chimney graft is defined as a bare or covered stent that is placed parallel to the main stent graft to preserve blood flow to the supra-aortic arch vessel, which is covered to achieve the proper landing and sealing zone. Since the procedure was introduced, the chimney graft has been successfully applied to preserve the blood flow of the carotid, subclavian, renal and superior mesenteric arteries during endovascular treatment of aortic disease.6)7) It has been used as the rescue procedure for accidental overstenting of the carotid, subclavian, renal and superior mesenteric arteries during endovascular treatment of aortic disease, and it has also been used for patients with descending thoracic aortic dissection to overcome the short landing zone using cut-down of the subclavian artery and the carotid artery.8) There are a number of differences between the classical chimney graft and our "modified chimney" technique. We intentionally cover two thirds of the left carotid artery with the stent graft, select the left carotid artery via the other femoral artery and then place the stent into the left carotid artery without cut-down of the left carotid artery. We named this technical modification the modified chimney technique. We report here on our experience with the modified chimney technique used to preserve the blood flow to a supra-aortic arch vessel in 2 patients with ruptured thoracic aortic dissection and thoracic aortic aneurysm.
A 49-year-old female experiencing untreated hypertension for several years presented with severe chest and back pain. A CT scan was performed and revealed an aortic dissection (Stanford type B) and an intimal flap was noted immediately distal from the origin of the left subclavian artery. After 4 days of medical management, her urine output decreased and both femoral pulses were weakened. Another CT scan was performed, showing that the aortic dissection had worsened and there was nearly total occlusion of the mid-aorta (Fig. 1). We recommended aortic repair operation, which the patient and her family refused. TEVAR was recommended as another treatment option to cover the intimal flap of the dissection. To acquire the proper proximal landing zone, her left subclavian artery and two thirds of the origin of the left common carotid artery would be covered by the stent graft. For the occluded left subclavian artery, revascularization can then be selectively considered in a staged approach if left arm pain, claudication and subclavian steal syndrome develop. The modified chimney technique was planned to preserve the blood flow of the left common carotid artery. The patient was taken to the cardiac catheterization laboratory and a left femoral arteriotomy was performed under general anesthesia. The 035 inch wire was placed in the ascending aorta and the left subclavian artery through the left radial artery approach. This wire is important to rescue the left carotid artery flow. If an aortic stent graft totally covers the left carotid artery or if it is difficult to select the left carotid artery with a catheter, then we can perform emergency balloon dilatation and place a chimney graft stent from the left subclavian artery to the aorta via this wire. The deployment of a 38×150 mm SEAL aortic stent graft (S&G Biotech, Seongnam, Korea) was performed so that the proximal part of the stent graft covered two thirds of the ostium of the left common carotid artery. After selection of the left common carotid artery using a 5 Fr Judkin right catheter (Cordis, Hialeah, FL, USA) through the gap between the stent graft and the left carotid artery, we passed a 035 inch Terumo wire (Terumo, Tokyo, Japan) into the left carotid artery through the right femoral artery and exchanged it with an Amplatz stiff wire. Then, an 8×60 mm SMART nitinol stent (Cordis, Hialeah, FL, USA) was deployed into the left common carotid artery. A final angiogram showed excellent results with good flow to both the thoracic aorta and the left common carotid artery. No endoleak was noted (Fig. 2). The occluded true lumen was re-expanded and the false lumen was not seen. The mean pressure gradient between the thoracic and abdominal aorta was decreased to 20 mm Hg from 90 mm Hg immediately after TEVAR (Fig. 2). After 18 months there has been no endoleak, restenosis of the stent in the left carotid artery or other complications.
A 75-year-old male with hypertension and medically treated aortic dissection that occurred several years ago again presented with severe chest and back pain. A CT scan revealed an aortic dissection (Stanford type B) combined with a massive aneurysmal dilatation, and the intimal tear site was nearly 2 cm from the left suclavian artery. Because the aortic aneurysm involved an aortic arch, the proximal landing zone was less than 1 cm wide (Fig. 3). Because of his age and the characteristics of the lesion, there was the possibility of further operation related complication (perioperative mortality, morbidity, stroke, paraplegia etc.) and the patient did not want open surgery; we therefore decided to perform TEVAR rather than open surgery. The patient was taken to the cardiac catheterization laboratory and an arteriotomy for the left femoral artery was performed under general anesthesia. The 035 inch wire was placed in the ascending aorta and the left subclavian artery through the left radial approach. The aortogram confirmed a large aortic aneurysm adjacent to the origin of the left subclavian artery. Two thirds of the origin of the left common carotid artery was covered by the proximal part of a 40×160 mm SEAL thoracic aortic stent (S&G biotech, Seongnam, Korea) to achieve an adequate landing zone. Another 40×130 mm SEAL thoracic aortic stent (S&G biotech, Seongnam, Korea) was deployed in the first aortic stent graft with a 7 cm overlapped segment to protect against disconnection of the aortic stent grafts. After selection of the left common carotid artery using a 5 Fr Judkin right catheter through the gap between the stent graft and the left carotid artery, we passed a 035 inch Amplatz stiff wire into the left carotid artery through the right femoral artery. An 8×60 mm SMART nitinol stent (Cordis, Hialeah, FL, USA) was then deployed into the left common carotid artery. The final angiogram showed excellent results with good flow to both the thoracic aorta and the left common carotid artery. No endoleak was noted (Fig. 4). On the clinical follow-up of this patient (20 months to date), the patient has had no problems related to the graft and the follow-up CT scan showed no sign of malfunctioning grafts, restenosis of the stent in the left carotid artery or other complications.
Over 40% of the patients who have undergone TEVAR have pathology that starts near the left subclavian artery.9) In these circumstances, the aortic stent grafts are currently typically placed over the origin of the left subclavian artery, thereby occluding the aortic arch vessel. Published reports have shown that the baseline risks of adverse outcomes in patients who have TEVAR and left subclavian artery coverage are 6% for arm ischemia, 4% for spinal cord ischemia, 2% for vertebrobasilar ischemia, 5% for anterior circulation stroke and 6% for death. Despite these adverse outcomes (whereby the necessity of left subclavian artery revascularization is controversial), most practitioners generally agree to perform left subclavian artery revascularization in certain situations, and particularly when there is a dominant left vertebral artery (60%), a previous left internal mammary coronary artery bypass graft or when the distal right vertebral segment is absent.10) In many circumstances, individualized care must be tailored using clinical judgment. Routine left subclavian artery revascularization may not be possible in some patients in whom ruptured aortic dissection or a ruptured aortic aneurysm requires immediate TEVAR or when the proper surgical expertise is not available. In our cases, the patients were in an emergency situation, the aortogram performed during the procedure showed no dominant left vertebral artery and the patients had not undergone a previous coronary artery bypass graft operation. It is reasonable to suggest that the left subclavian artery revascularization should be planned in a staged approach when symptoms (arm pain, claudication and subclavian steal syndrome) occur. For the modified chimney technique, we consistently placed the 035 wire in the ascending aorta and then in the left subclavian artery through the left radial approach. This wire is important to rescue the left carotid artery flow. If an aortic stent graft totally covers the left carotid artery or it is difficult to select the left carotid artery with the catheter, then we can perform emergency balloon dilatation and a classic chimney graft stent from the left subclavian artery to the aorta via this wire. The wire could be useful for left subclavian artery revascularization if this is needed.
In the modified chimney technique, the chimney stent is inserted via the femoral artery and not via the carotid artery. There is no need to puncture or cut down the supra-aortic arch vessels as this procedure is inconvenient for the operator and there could be complications during the procedure such as bleeding, pneumothorax and stroke. Using a 5 Fr catheter (Judkin Right 5 Fr) through the other femoral artery, super selection of the left common carotid artery was easily performed and the 035 inch Terumo guidewire was placed into the left common carotid artery. Then, the guide wire was exchanged with a 035 inch Amplatz stiff guide wire. The self expandable stent was inserted via the other femoral artery, and the chimney stent was placed protrusively into the aorta across the aortic stent graft to preserve the left carotid flow. Actually, we selected the left carotid artery within 5 minutes. This was possible and easily performed because the proximal end of the main stent covered only two thirds of the left common carotid artery orifice. We were able to acquire an adequate proximal landing zone by the modified chimney technique.
The classic chimney graft technique had a low rate of adverse events,11) yet it is possible that the chimney graft technique has an increased risk of endoleak compared to that of the conventional TEVAR. This is because the chimney graft stent was inserted parallel to the proximal end of the main stent. An endoleak could possibly occur between the two stents. The modified chimney technique is similar to the two stent insertion technique for the coronary bifurcation, and the chimney stent was not inserted exactly parallel to the main stent. Thus theoretically, there is less chance for blood to leak between the two stents. However, further studies will be required to firmly determine this.
The modified chimney technique could be useful when the aortic main stent graft accidentally covers the supra-aortic branch vessels. As described above, there are advantages in using the modified chimney technique, including: using the same entry site of the main stent; there is no need to puncture or cut down the carotid artery; a short procedure time; and fewer complications such as bleeding, pneumothorax and stroke. However, when the main stent accidentally covers the entire orifice of the left common carotid artery and the aortogram shows no blood flow to the left common carotid artery, then the retrograde approach needs to be performed via the left carotid artery.
The modified chimney technique could be useful due to various aspects. However, it is unable to become the next step of TEVAR because the modified chimney technique cannot be applied in all the patients with thoracic aortic pathology. The branched and fenestrated aortic stent graft may be the next approach if it is convenient, less complicated and it becomes available as an off-the-shelf device. This may become clear after further studies are carried out.
In conclusion, While TEVAR is a treatment option for thoracic aortic pathology, it does have limitations such as anatomical problems. The modified chimney technique could be used as an alternative option when aortic pathology is involved near the supra-aortic arch vessels in an emergency situation, and as a rescue procedure when a stent graft covers the left carotid artery accidentally.
Figures and Tables
Fig. 1
CT showed a Stanford type B aortic dissection and intramural hematoma at the thoraco-abdominal aorta (A-D). An intimal flap was noted just distal from the left subclavian artery (white arrow in E and F). The intramural hematoma extended to the origin of the renal artery (C).
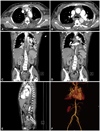
Fig. 2
The overall procedure of the patient. A: the aortogram before stent graft deployment; the proximal entry tear was distal to the LSCA. B: the aortogram after stent graft deployment; the proximal entry tear was completely sealed. The origins of the left carotid artery and LSCA were covered because of the short proximal landing zone. C: selection of the LCCA with a Judkin right 5 Fr catheter through the right femoral artery. D: stent deployment in the LCCA by the chimney graft technique. E: the aortogram after successful stent graft deployment; the occluded true lumen was reexpanded and the false lumen was not seen. The mean pressure gradient between the thoracic and abdominal aorta was decreased to 20 mm Hg from 90 mm Hg after TEVAR. There was no endoleak. TEVAR: thoracic endovascular aortic repair, LSCA: left subclavian artery, LCCA: left common carotid artery.
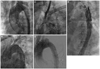
Fig. 3
The CT scan revealed a Stanford type B aortic dissection combined with a huge aneurysmal dilatation for which the entry site was the aortic arch level and the aneurysm extended to the infrarenal level and ran to the common iliac artery.
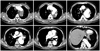
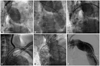
Fig. 4
The overall procedure of the patient. A and B: the aortogram before stent graft deployment; the proximal entry tear was distal to the LSCA and there was a huge aneurysm. C: the aortogram after stent graft deployment; the huge aneurysm was completely sealed. The origins of the left carotid artery and LSCA were covered because of the short proximal landing zone. D: selection of the LCCA with a Judkin right 5 Fr catheter through the right femoral artery. E: stent deployment in the LCCA by the chimney graft technique. F: the aortogram after successful stent graft deployment: There was no endoleak. LSCA: left subclavian artery, LCCA: left common carotid artery.
Acknowledgments
This study was supported by a grant from the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A084177).
References
1. Cheng D, Martin J, Shennib H, et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J Am Coll Cardiol. 2010. 55:986–1001.
2. Malina M, Resch T, Sonesson B. EVAR and complex anatomy: an update on fenestrated and branched stent grafts. Scand J Surg. 2008. 97:195–204.
3. Chuter TA, Schneider DB. Endovascular repair of the aortic arch. Perspect Vasc Surg Endovasc Ther. 2007. 19:188–192.
4. Sonesson B, Resch T, Allers M, Malina M. Endovascular total aortic arch replacement by in situ stent graft fenestration technique. J Vasc Surg. 2009. 49:1589–1591.
5. Criado FJ. Chimney grafts and bare stents: aortic branch preservation revisited. J Endovasc Ther. 2007. 14:823–824.
6. Greenberg RK, Clair D, Srivastava S, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003. 38:990–996.
7. Allaqaband S, Jan MF, Bajwa T. "The chimney graft"-a simple technique for endovascular repair of complex juxtarenal abdominal aortic aneurysms in no-option patients. Catheter Cardiovasc Interv. 2010. 75:1111–1115.
8. Ohrlander T, Sonesson B, Ivancev K, Resch T, Dias N, Malina M. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther. 2008. 15:427–432.
9. Feezor RJ, Martin TD, Hess PJ, et al. Risk factors for perioperative stroke during thoracic endovascular aortic repairs (TEVAR). J Endovasc Ther. 2007. 14:568–573.
10. Rizvi AZ, Murad MH, Fairman RM, Erwin PJ, Montori VM. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg. 2009. 50:1159–1169.
11. Larzon T, Gruber G, Friberg O, Geijer H, Norgren L. Experiences of intentional carotid stenting in endovascular repair of aortic arch aneurysms: two case reports. Eur J Vasc Endovasc Surg. 2005. 30:147–151.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download