Abstract
Contrast-induced nephropathy (CIN) affects in-hospital, short- and long-term morbidity and mortality. It also leads to prolonged hospital stay and increased medical cost. Given the potential clinical severity of CIN, there has been considerable interest in the development of preventative strategies to reduce the risk of contrast-induced renal deterioration in at-risk populations. A number of pharmacologic and mechanical preventive measures have been attempted, but no method other than adequate periprocedural hydration has been conclusively successful. Since its introduction in 2000, N-acetylcysteine (NAC) has been widely investigated, albeit with conflicting findings for its nephroprotection capability in patients receiving contrast media procedures. However, there is still virtually no definitive evidence of effectiveness of NAC. Although the exact mechanism responsible for the protective action of NAC from renal function deterioration remains unclear, the antioxidant and vasodilatory properties of NAC have been suggested as the main mechanisms. This review summarizes the current status of NAC as a potential agent to prevent renal functional deterioration and its limitations.
Contrast-induced nephropathy (CIN) is usually defined as an acute decline in renal function, expressed as a relative increase in serum creatinine (SCr) concentration of at least 25% or an absolute increase in SCr of 0.5 mg/dL (44.2 µmol/L) in the absence of other etiologies.1) CIN is a significant problem in clinical practice, but also one that is often unrecognized. In 12% of cases, it is the third leading cause of hospital-acquired acute renal failure2) and is associated with 36% mortality in patients who require in-hospital dialysis with 19% survival at 2 years.3)4) When there are no risk factors, the incidence of CIN is low (<5%).5) In modern clinical practice, the characteristic demographic change is a growing elderly population, often patients with cardiovascular and renal comorbidities. Such a change is associated with a greater number of contrast-enhanced invasive diagnostic and interventional coronary procedures, which increases the likelihood of further renal deterioration due to radiocontrast.6)7) In view of the high morbidity and mortality associated with CIN, measures taken to prevent or minimize its occurrence in patients at-risk are extremely important.
Intravascular volume expansion with saline or sodium bicarbonate and low- or iso-osmolar contrast agents, such as iodixanol, are all associated with a decreased rate of CIN.8) Although low- or iso-osmolar contrast agents with adequate hydration reduces the risk for CIN by two-thirds, they did not totally eliminate the risk.9) Therefore, many clinical trials have evaluated various pharmacological agents and periprocedural factors in an effort to identify successful strategies for reducing the risk.10-14) Pharmacological agents evaluated for their potential role in reducing CIN risk include vasodilators and antioxidants, a reflection of the current understanding of the pathophysiology of contrast-induced renal injury. Vasodilatory intervention with dopamine,15) fenoldopam,16) calcium channel blockers,17) and theophylline18) have been investigated, but results are inconsistent, calling for additional large, prospective, randomized studies.
N-acetylcysteine (NAC), a thiol-containing antioxidant, is one of the antioxidants that has been investigated extensively as an agent for CIN prevention.19)20) Tepel and colleagues reported in 2000 an almost 90% relative risk reduction in the incidence of CIN in patients with chronic kidney disease (CKD) given NAC added to prophylactic hydration after intravenous administration of contrast media for elective computed tomography examinations. Moreover, greater efficacy was found with increased NAC dosing (1,200 mg vs. 600 mg, twice daily),21) consistent with the suggestion that the antioxidant effects of NAC are dose-dependent.22) These findings provided the rationale to investigate use of NAC for prevention of CIN in patients with CKD undergoing cardiovascular procedures and receiving intra-arterial contrast agents.
However, despite these positive findings, the true efficacy of NAC for CIN prevention remains unclear given several discordant meta-analyses, mainly as a result of a high degree of heterogeneity among trials.23-29)
Although not having been consistently shown to be effective,30-32) NAC is often recommended and used in an effort to reduce the rate of CIN due to its ease in administration, easy availability, low cost, and few side effects.33)34)
This review focuses on the current status of NAC and intends to guide to renal protection in patients receiving contrast procedures.
Although there have been a number of in-vivo studies and in vitro animal studies, which have the potential to be extrapolated to humans, the pathogenesis of CIN in humans is still unclear.
The main mechanisms of CIN are suggested as a direct renal tubular toxicity and renal medullary ischemia.35) Administration of contrast medium increases the production of nephrotoxic oxygen free radicals.36)37) There is a large body of evidence that reactive oxygen species have a role in the renal damage caused by radio-contrast agents.38-40)
NAC is an antioxidant that attenuates ischemic renal failure in animal studies.41)42) Besides scavenging oxygen free radicals that mediate cell necrosis after myocardial infarction43) and after angioplasty,44) NAC may act as an antioxidant to inhibit ischemic cell death in the kidney.
Antioxidants such NAC and ascorbic acid protect tubule cells from apoptosis related to reactive oxygen species.45) The cytotoxicity of contrast media (CM) on human embryonic kidney (HEK 293), porcine proximal renal tubular (LLC-PK1), and canine Madin-Darby distal tubular renal (MDCK) cells has been evaluated, and the effectiveness of various antioxidant compounds like NAC, ascorbic acid, and sodium bicarbonate in preventing CM cytotoxicity has been studied. Both low- and iso-osmolar CM induce a dose-dependent renal cell apoptosis. NAC and ascorbic acid, but not sodium bicarbonate, prevents this CM-induced apoptosis.
In a similar study comparing three antioxidants (NAC, ascorbic acid, and probucol), NAC pretreatment significantly improved HEK cell viability as compared with control (p<0.001). Probucol or ascorbic acid pretreatment does not show reduction of cell death caused by CM.46) This result may indicate that NAC is a better antioxidant than ascorbic acid with regard to the CIN prevention.47) The comparison of NAC with ascorbic acid is important, because the combination of these two measures has no additive effect in reducing CIN rate as compared with use of NAC alone.48) This may be mainly attributed to their shared similar mechanism of oxygen free radical scavenging.
A recently published large animal study50) also supports the benefit of NAC in CIN prevention. In the study, intracoronary radiographic CM combined with NAC protected renal function and reduced myocardial infarction size in a pig model of ischemia and reperfusion. Histopathologic analysis of the myocardium revealed a reduction in programmed cell death by NAC-enhanced contrast medium that may explain the increase in ischemia tolerance. NAC-enhanced contrast medium administration blunted the rise in SCr levels by 60% and decreased renal tubule cell apoptosis.
Contrast media also reduce renal function by altering renal hemodynamics as well and NAC has the potential to prevent CIN by improving this. Reduced blood flow in the renal outer medulla, which has a high demand for oxygen and is very vulnerable to hypoxia, might result from increased perivascular hydrostatic pressure, high viscosity, or changes in vasoactive substances such as nitric oxide, adenosine, and endothelin by contrast dye.49)50)
In addition to the CM injected, volume depletion has been recognized as a predisposing factor for CIN. In a study,52) saline or high-osmolar CM was injected into volume-depleted rats and the glomerular filtration rate (GFR) and renal plasma flow rate were measured 24 hours later. Both rates were reduced by 50% in contrast-injected rats, as compared with saline-injected, water depleted rats. CM did not induce renal dysfunction and enhance lipid peroxidation in non-water depleted rats. However in water depleted contrast-treated rats, specific products of membrane lipid peroxidation, namely phosphatidylcholine and phosphatidylethanolamine hydroperoxide, which are markers of oxidative stress, were more than two-fold higher than saline-treated water depleted rats. Therefore, the contrast medium appeared to induce oxidant-mediated injury only in water depleted rats.51)
Some studies have suggested that NAC has vasodilatory properties. In one study with 14 dogs, NAC (150 mg/kg, followed by a 20 mg/kg/hr infusion) increased blood flow in mesenteric, renal and especially femoral arteries than control group.52) Oxygen delivery and oxygen-uptake were higher in the NAC-treated dogs than in the control animals. Moreover, NAC ameliorated ischemia in kidney by general properties as an antioxidant or a possible interaction with NAC and NO.53)
Mitogen activated protein kinases (MAPKs) are integral components of the parallel MAPK cascades activated in response to a variety of cellular stress inducing ischemia/ATP depletion and inflammatory cytokines. Members of the MAPK family, in particular c-Jun N-terminal kinase (JNK), are activated in the kidney following ischemia/reperfusion. Pretreatment with a combination of NAC with sodium nitroprusside and phosphoramidon can completely inhibit MAPK.54) NAC has also been reported to block the expression of vascular-cell adhesion molecule 1 and the activation of nuclear factor-κB in glomerular mesangial cells,55) which implies that NAC may protect kidney function by blocking a signal in the pathogenesis of glomerular mesangial cell disorders.
The first human study with respect to kidney function dealt with hepatic failure from drug poisoning. Early administration of NAC prevented a reduction in renal function in patients with acetaminophen poisoning who had liver failure, and NAC may have improved renal function in patients with the hepatorenal syndrome.56-59)
A small clinical study dealing examining NAC in the prevention of CIN was published in 2000.60) The investigators enrolled 83 patients with chronic renal failure (mean SCr, 2.4 mg/dL) who were undergoing computed tomography. NAC (600 mg, orally, twice daily before and after CM) was administered with concomitant saline hydration. CIN occurred 10-times less, 2% in the NAC group and 21% in the control group {p=0.01, relative risk, 0.1; 95% confidence interval (0.02-0.9)}. The publication of these results spurred a myriad of clinical trial, which produced mixed data, even in a meta-analysis.
Patients with acute myocardial infarction treated with primary percutaneous coronary intervention (PCI) are at higher risk of CIN than those undergoing elective PCI.61) In patients with acute myocardial infarction, several conditions may contribute to the development of renal dysfunction. Impaired systemic perfusion due to depressed left ventricular function, a larger dose of contrast medium, and the impossibility of starting renal prophylaxis before exposure to contrast medium may be involved.
The first study evaluating the effect of NAC on CIN in the setting of primary PCI in patient with ST elevation of myocardial infarction was published in 2006. One hundred and sixteen patients were randomized to receive NAC (600 mg intravenous bolus before CAG and 600 mg orally twice 48 hours after primary PCI), 119 were randomized to receive a double dose of NAC (1,200 mg, same schedule) and 119 were randomized to placebo. SCr increase ≥25% was regarded as confirmation of the development of CIN.62) CIN occurred in a dose responsive manner, with 5% in the double dose NAC group, 15% in the usual dose group, and 33% in the placebo group (p<0.001) (Fig. 1). In-hospital mortality was higher in patients with CIN than those without it (26% vs. 1%, p<0.002). Moreover, the rate for the composite end point of death, acute renal failure requiring temporary renal-replacement therapy, or the need for mechanical ventilation was significantly lower in the double and usual dose NAC groups than placebo, in a dose-dependent manner (5%, 7%, and 17% in the three groups, respectively; p=0.002).
Recently contradictory study results have been published in the same clinical setting with a use of only intravenous NAC in patients with ST-segment elevation myocardial infarction undergoing primary PCI. Activated oxygen protein products and oxidized low-density lipoprotein as markers for oxidative stress were measured with an administration of NAC and placebo. These markers were reduced by as much as 20% in the NAC group (intravenous bolus of 1,200 mg before PCI and 1,200 mg intravenously twice daily for the 48 hours after PCI) (p<0.05), whereas no change was detected in the placebo group. However, the data did not indicate an additional clinical benefit to placebo with respect to CIN. The primary end point, CIN, occurred in 14% of the NAC group and 20% of the placebo group (p=0.28).63)
Another study also demonstrated negative results with intravenous NAC. In the study, 398 acute coronary syndrome patients were randomized to receive an intravenous double dose of NAC (n=205; 1,200 mg bolus followed by 200 mg/hr for 24 hours) and placebo (n=192). There was no difference for the primary end point of CIN defined as an increase in SCr concentration ≥25% above the baseline level within 72 hours of the administration of IV contrast CIN in 16% of the NAC group and in 13% of the placebo group (p=0.40). Recent small-scale studies showed similar negative results.65)
The discrepancy of results may reflect a low contrast dose and, for the negative results, somewhat preserved renal function. Therefore, it is premature to recommend the strategy of intravenous NAC or oral NAC followed by intravenous NAC in primary angioplasty until more definitive data emerges.
Some investigators have argued that NAC influences SCr, the surrogate marker of GFR, without affecting true GFR per se. In an uncontrolled study of healthy volunteers, NAC reduced SCr, but not cystatin C, prompting the conclusion that NAC might affect SCr independent of the GFR.66) However, this effect of NAC has not been investigated in the setting of renal insufficiency. Some contradictory results have been reported. One study that assayed both SCr and cystatin C reported a significant correlation between the parameters at baseline and a stronger correlation 48 hours after contrast exposure in the NAC treatment group.67) Similarly, in randomized trials designed to prevent acute renal deterioration in subjects with renal insufficiency that used peri-operative high-dose NAC infusion (300 mg/kg intravenously), SCr and cystatin C demonstrated concordant response. There was same pattern of creatinine-lowering effect, urinary creatinine excretion and plasma creatinine/plasma cyctatin C ratio for the NAC and placebo groups.68)69) Therefore, it is unlikely that NAC reduced the SCr independent of renal function. It must be noted that the SCr level is an imperfect surrogate outcome measure for nephropathy, and that protection against nephropathy based on altered SCr level has not been confirmed using other measures.
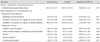
In a recent large, multicenter, randomized clinical trial including 2,308 patients, coronary angiography was performed in patients with at least one risk factor of CIN (70 years of age, renal failure, diabetes mellitus, full term for HF, hypertension). Patients were allocated to NAC (1,200 mg orally twice daily) before and after the contrast procedure. NAC did not reduce CIN or clinical outcomes. CIN occurred at a same rate, 12.7%, in NAC and placebo groups. Clinical outcomes of mortality or need for dialysis at 30 days was 2.2% and 2.3% in the NAC and placebo group, respectively, with hazard ratio of 0.97 {95% confidence interval (CI), 0.56-1.69; p=0.92} (Table 3).70) In subgroups categorized by basal renal function, contrast dose, age, and diabetes status, there were no differences with respect to CIN rate and cardiovascular events between the NAC and placebo groups.
It is the largest randomized relevant clinical trial performed to date, and was well designed and well conducted. Moreover its statistical power and analysis bring confidence to the results. Considering that the previous conflicting results of small trials and some meta-analyses were rooted in the study heterogeneity and publication bias, where negative results are less likely to be published, this multicenter prospective randomized clinical trial is of great interest and significance. Some expert expressed that this trial will influence clinical practice by dissuading use of NAC for the purpose of renal protection.71)
In light of this latest trial, NAC may well fall out of favor as a routine treatment option until new evidence from studies involving longer duration of use and much higher doses are published. In the interim, a recommendation is to adequately expand intravascular volume with saline at least 12 hours before and after the contrast procedure (Table 1 and 2).
Several meta-analyses were conducted to resolve difficult issue of true effectiveness of NAC. However, the results have been disappointingly equivocal, given the limitations of the small studies on which the analyses were are based.
In 2003, a meta-analysis including seven trials involving 805 patients demonstrated that administration of NAC and hydration significantly reduced the relative risk of contrast nephropathy by 56% {0.435 (95% CI 0.215-0.879), p=0.02} as compared with periprocedural hydration alone in patients with chronic renal insufficiency. However, significant heterogeneity was indicated (overall CIN incidence in NAC group, 2-26%). Other meta-analyses confirmed the heterogeneity in these trials related to publication bias, dose of the agent, cohort studied, and definition of outcome.
Dose may matter. In a recent meta-analysis that included 16 trials involving 1,677 high-risk patients with renal insufficiency using higher NAC doses, a 64% decrease in the likelihood of acute renal failure was evident. This study demonstrated no heterogeneity and no evidence of publication bias (p=0.34). Most studies of NAC's prophylactic potential have used lower doses (e.g., 600 mg twice a day), raising the possibility that the failure to consistently see a benefit may have resulted from under-dosing.
Identified patient and study characteristics may be responsible for some, but not all, of this inconsistency. So, at present, a definitive conclusion cannot be made.
There have been mixed data on whether prophylactic oral and IV NAC administration reduces the incidence of CIN in small trials and even in meta-analyses, although its use is generally recommended, given its low cost, easy availability, and favorable side effect profile. Moreover, evidence of any improvement in clinical outcomes at long-term follow-up is still lacking, despite beneficial effects in CIN prevention.
Research on NAC and the incidence of CIN is too inconsistent at present to warrant a conclusion on efficacy or a recommendation for its routine use. A large, randomized, placebo-controlled trial, a pooled analysis of patient-level data, or both may resolve this issue.
The efficacy of NAC for preventing CIN remains unproven. Future studies should not be based on a primary end point of changed SCr level. Instead, the efficacy and safety of NAC should be sought in critically ill patients at risk for CIN.
Figures and Tables
Fig. 1
Linear trends for low rate of contrast-induced nephropathy by dose of NAC and by normal and reduced creatinine clearance rates (>60 mL/min and ≤60 mL/min, respectively). The p values refer to comparisons among the placebo group, the group receiving a standard dose of NAC, and the group receiving a double dose of NAC (calculated with the use of chi-square for trend). No significant interactions were found between groups and creatinine clearance (p=0.25).73) NAC: N-acetylcysteine, CCr: creatinine clearance rate.

Table 1
Recommendations for prevention of contrast-induced nephropathy72)

*Class of recommendation, †Level of evidence, ‡Recommendation pertains to the type of contrast. CKD: chronic kidney disease, OMT: optimal medical therapy, ACE: angiotensin-converting enzyme, EF: ejection fraction, NYHA: New York Heart Association, LOCM: low osmolar contrast media, IOCM: iso-osmolar contrast media, i.v.: intravenous, PCI: percutaneous coronary intervention
Table 2
Recommendations of interventions commonly used to reduce the risk of contrast-induced nephropathy74)

Table 3
Contrast-induced nephropathy rate according to N-acetylcysteine and placebo with no differences72)
References
1. Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000. 11:177–182.
2. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002. 39:930–936.
3. McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997. 103:368–375.
4. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998. 32:5 Suppl 3. S112–S119.
5. Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002. 105:2259–2264.
6. Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990. 89:615–620.
7. Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial: the Iohexol Cooperative Study. Kidney Int. 1995. 47:254–261.
8. Jo SH, Youn TJ, Koo BK, et al. Renal toxicity evaluation and comparison between visipaque (iodixanol) and hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER Study: a randomized controlled trial. J Am Coll Cardiol. 2006. 48:924–930.
9. McCullough PA, Bertrand ME, Brinker JA, Stacul F. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol. 2006. 48:692–699.
10. Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004. 291:2328–2334.
11. Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002. 162:329–336.
12. Stevens MA, McCullough PA, Tobin KJ, et al. A prospective randomiz-ed trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. Study: Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol. 1999. 33:403–411.
13. Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994. 331:1416–1420.
14. Weinstein JM, Heyman S, Brezis M. Potential deleterious effect of furosemide in radiocontrast nephropathy. Nephron. 1992. 62:413–415.
15. Gare M, Haviv YS, Ben-Yehuda A, et al. The renal effect of low-dose dopamine in high-risk patients undergoing coronary angiography. J Am Coll Cardiol. 1999. 34:1682–1688.
16. Stone GW, McCullough PA, Tumlin JA, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA. 2003. 290:2284–2291.
17. Russo D, Testa A, Della Volpe L, Sansone G. Randomised prospective study on renal effects of two different contrast media in humans: protective role of a calcium channel blocker. Nephron. 1990. 55:254–257.
18. Huber W, Schipek C, Ilgmann K, et al. Effectiveness of theophylline prophylaxis of renal impairment after coronary angiography in patients with chronic renal insufficiency. Am J Cardiol. 2003. 91:1157–1162.
19. McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997. 103:368–375.
20. Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000. 343:180–184.
21. Briguori C, Colombo A, Violante A, et al. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J. 2004. 25:206–211.
22. Cotgreave I, Moldéus P, Schuppe I. The metabolism of N-acetylcysteine by human endothelial cells. Biochem Pharmacol. 1991. 42:13–16.
23. Birck R, Krzossok S, Markowetz F, Schnülle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003. 362:598–603.
24. Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008. 148:284–294.
25. Liu R, Nair D, Ix J, Moore DH, Bent S. N-acetylcysteine for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis. J Gen Intern Med. 2005. 20:193–200.
26. Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int. 2004. 65:1366–1374.
27. Nallamothu BK, Shojania KG, Saint S, et al. Is acetylcysteine effective in preventing contrast-related nephropathy? a meta-analysis. Am J Med. 2004. 117:938–947.
28. Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009. 122:874.e9–874.e15.
29. Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 2007. 5:32.
30. Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006. 113:1799–1806.
31. Kshirsagar AV, Poole C, Mottl A, et al. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004. 15:761–769.
32. Ozcan EE, Guneri S, Akdeniz B, et al. Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy: a comparison of 3 regimens for protecting contrast-induced neph-ropathy in patients undergoing coronary procedures: a single-center prospective controlled trial. Am Heart J. 2007. 154:539–544.
33. Barrett BJ, Parfrey PS. Prevention of nephrotoxicity induced by radiocontrast agents. N Engl J Med. 1994. 331:1449–1450.
34. Pannu N, Wiebe N, Tonelli M. Alberta Kidney Disease Network. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006. 295:2765–2779.
35. Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol. 1994. 5:125–137.
36. Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis. 1997. 29:465–477.
37. Bakris GL, Lass N, Gaber AO, Jones JD, Burnett JC Jr. Radiocontrast medium-induced declines in renal function: a role for oxygen free radicals. Am J Physiol. 1990. 258(1 Pt 2):F115–F120.
38. Messana JM, Cieslinski DA, Humes HD. Comparison of toxicity of radiocontrast agents to renal tubule cells in vitro. Ren Fail. 1990. 12:75–82.
39. Katholi RE, Woods WT Jr, Taylor GJ, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998. 32:64–71.
40. Yoshioka T, Fogo A, Beckman JK. Reduced activity of antioxidant enzymes underlies contrast media-induced renal injury in volume depletion. Kidney Int. 1992. 41:1008–1015.
41. DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol. 1997. 272(3 Pt 2):F292–F298.
42. Salom MG, Ramírez P, Carbonell LF, et al. Protective effect of N-acetyl-L-cysteine on the renal failure induced by inferior vena cava occlusion. Transplantation. 1998. 65:1315–1321.
43. Arstall MA, Yang J, Stafford I, Betts WH, Horowitz JD. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction: safety and biochemical effects. Circulation. 1995. 92:2855–2862.
44. Pollman MJ, Hall JL, Gibbons GH. Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury: influence of redox state and cell phenotype. Circ Res. 1999. 84:113–121.
45. Romano G, Briguori C, Quintavalle C, et al. Contrast agents and renal cell apoptosis. Eur Heart J. 2008. 29:2569–2576.
46. Lee HC, Sheu SH, Liu IH, et al. Impact of short-duration administration of N-acetylcysteine, probucol and ascorbic acid on contrast-induced cytotoxicity. J Nephrol. 2011. 04. 20. [Epub]. http://dx.doi.org/10.5301/JN.2011.7741.
47. Jo SH, Koo BK, Park JS, et al. N-acetylcysteine versus AScorbic acid for preventing contrast-Induced nephropathy in patients with renal insufficiency undergoing coronary angiography NASPI study-a prospective randomized controlled trial. Am Heart J. 2009. 157:576–583.
48. Briguori C, Airoldi F, D'Andrea D, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007. 115:1211–1217.
49. Solomon R. Contrast-medium-induced acute renal failure. Kidney Int. 1998. 53:230–242.
50. Heyman SN, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: a role for medullary hypoxia. Invest Radiol. 1999. 34:685–691.
51. Yoshioka T, Fogo A, Beckman JK. Reduced activity of antioxidant enzymes underlies contrast media-induced renal injury in volume depletion. Kidney Int. 1992. 41:1008–1015.
52. Zhang H, Spapen H, Nguyen DN, Rogiers P, Bakker J, Vincent JL. Effects of N-acetyl-L-cysteine on regional blood flow during endotoxic shock. Eur Surg Res. 1995. 27:292–300.
53. Salom MG, Ramírez P, Carbonell LF, et al. Protective effect of N-acetyl-L-cysteine on the renal failure induced by inferior vena cava occlusion. Transplantation. 1998. 65:1315–1321.
54. Mehta A, Sekhon CP, Giri S, Orak JK, Singh AK. Attenuation of ischemia/reperfusion induced MAP kinases by N-acetyl cysteine, sodium nitroprusside and phosphoramidon. Mol Cell Biochem. 2002. 240:19–29.
55. Khachigian LM, Collins T, Fries JW. N-acetyl cysteine blocks mesangial VCAM-1 and NF-kappa B expression in vivo. Am J Pathol. 1997. 151:1225–1229.
56. Brady HR, Singer GG. Acute renal failure. Lancet. 1995. 346:1533–1540.
57. Vale JA, Proudfoot AT. Paracetamol (acetaminophen) poisoning. Lancet. 1995. 346:547–552.
58. Holt S, Goodier D, Marley R, et al. Improvement in renal function in hepatorenal syndrome with N-acetylcysteine. Lancet. 1999. 353:294–295.
59. Izzedine H, Kheder-Elfekih R, Deray G, Thabut D. Endothelin-receptor antagonist/N-acetylcysteine combination in type 1 hepatorenal syndrome. J Hepatol. 2009. 50:1055–1056.
60. Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008. 148:284–294.
61. Sadeghi HM, Stone GW, Grines CL, et al. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003. 108:2769–2775.
62. Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006. 354:2773–2782.
63. Thiele H, Hildebrand L, Schirdewahn C, et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: the LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol. 2010. 55:2201–2209.
64. Jaffery Z, Verma A, White CJ, et al. A randomized trial of intravenous N-acetylcysteine to prevent contrast induced nephropathy in acute coronary syndromes. Catheter Cardiovasc Interv. 2011. 05. 03. [Epub]. http://dx.doi.org/10.1002/ccd.23157.
65. Tanaka A, Suzuki Y, Suzuki N, et al. Does N-acetylcysteine reduce the incidence of contrast-induced nephropathy and clinical events in patients undergoing primary angioplasty for acute myocardial infarction? Intern Med. 2011. 50:673–677.
66. Hoffmann U, Fischereder M, Krüger B, Drobnik W, Krämer BK. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004. 15:407–410.
67. Poletti PA, Saudan P, Platon A, et al. I.v. N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. AJR Am J Roentgenol. 2007. 189:687–692.
68. Ristikankare A, Kuitunen T, Kuitunen A, et al. Lack of renoprotective effect of i.v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth. 2006. 97:611–616.
69. Haase M, Haase-Fielitz A, Ratnaike S, et al. N-Acetylcysteine does not artifactually lower plasma creatinine concentration. Nephrol Dial Transplant. 2008. 23:1581–1587.
70. ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced Nephropathy Trial (ACT). Circulation. 2011. 124:1250–1259.
71. McCullough PA, Khambatta S, Jazrawi A. Minimizing the renal toxicity of iodinated contrast. Circulation. 2011. 124:1210–1211.
72. Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010. 31:2501–2555.
73. Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty 1. N Engl J Med. 2006. 354:2773–2782.
74. Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006. 354:379–386.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download