Abstract
A coronary aneurysm (CA) can occur in sirolimus-eluting stent (SES)-implanted coronary lesions. Although several possible mechanisms have been suggested, the precise pathogenesis of a CA in SES-implanted lesions is still unknown. We report a patient with Churg-Strauss syndrome who underwent successful percutaneous coronary intervention with SES and then experienced a CA in an SES-implanted coronary lesion. We describe the CA characteristics through the use of coronary angiography, coronary 64-multidetector computed tomography, and intravascular ultrasound and discuss the etiological factors for the CA in this patient.
The sirolimus-eluting stent (SES) has provided excellent clinical outcomes by significantly reducing the number of major adverse cardiac events.1)2) However, SESs can cause unexpected vascular complications, such as stent thrombosis3) and a coronary aneurysm (CA)4) at SES-implanted coronary lesions. Furthermore, systemic necrotizing vasculitis, such as Churg-Strauss syndrome (CSS), can involve the native coronary artery in the form of coronary ectasia or an aneurysm. Although the precise mechanisms of CA development at SES-implanted coronary lesions are still unclear, both SESs and CSS can precipitate a CA by weakening the coronary vessel walls.5) We report a case of CA at an SES-implanted coronary lesion in a patient with CSS and describe the lesion features using several imaging modalities.
A 43 year old man presented with ongoing resting chest pain for the past 2 hours. He had been diagnosed with CSS at another general hospital 4 months earlier. He met the diagnostic criteria for CSS6) with asthma, allergic sinusitis, hypereosinophilia, and systemic vasculitis of the skin, and was receiving oral prednisone and cyclophosphamide. He had no other coronary risk factors. An initial electrocardiogram showed an ST-segment elevation from the V2 to V6 leads. Diagnostic coronary angiography (CAG) revealed a total occlusion of the mid-left anterior descending (LAD) coronary artery (Fig. 1A). After a manual intracoronary aspiration thrombectomy (Export; Medtronic, Santa Rosa, CA, USA) at the mid LAD lesion (Fig. 1B), a subsequent CAG revealed diffuse irregular and significant stenosis from the mid to distal LAD with restoration of thrombolysis and myocardial infarction grade 3 (Fig. 1C). A successful primary percutaneous coronary intervention (PCI) was performed with 2 SESs (Cypher; Cordis Johnson and Johnson, Warren, NJ, USA) placed from the mid to proximal LAD (diameter×length: 3.0×33 mm, and 3.5×23 mm) at a pressure of 18 atm (Fig. 1D and E). The final CAG showed good angiographic results at the LAD lesion (Fig. 1F). The patient was discharged on the third day with no cardiac symptoms and was prescribed 75 mg clopidogrel and 100 mg aspirin daily.
A follow-up CAG was performed at 14 months after the primary PCI. We substituted coronary 64-multidetector computed tomography (MDCT) for conventional CAG. Three-dimensional (3-D) surface reconstruction images from the coronary 64-MDCT revealed good positioning of the two over-lapping SESs with no neointimal hyperplasia (Fig. 2A). Additionally, a volume-rendering image on coronary 64-MDCT showed no neointimal hyperplasia or stent malapposition (Fig. 2B) compared to the final result.
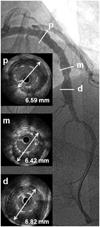
He was readmitted to our hospital 39 months later with atypical chest discomfort for the past 10 days. We performed a follow-up coronary 64-MDCT and CAG. The follow-up 3-D surface reconstruction view on coronary 64-MDCT disclosed a fusiform like diffuse enlargement of the LAD at the SES-implanted lesion. (Fig. 2C), and the volume-rendering image on coronary 64-MDCT showed a crescent-like shape to contrast dye filling in around the stent border (Fig. 2D). A follow-up CAG showed a diffuse multiple conglomerate contrast filling in along the stent border without instent restenosis (Fig. 2E). An intravascular ultrasound revealed significant gaps between the SES struts and the vessel wall and an enlargement of the external elastic membrane with a maximum diameter of 6.59 mm in the proximal portion, 6.42 mm in the mid portion, and 6.82 mm in the distal portion (Fig. 3). We then had the patient undergo an exercise treadmill test to confirm myocardial ischemia; however, the result was negative. Finally, we decided to treat the patient with medical therapy. The patient is under careful observation and is receiving dual antiplatelet therapy.
A CA is defined as a segment with a diameter greater than 1.5 times the normal adjacent artery segment and can be classified as fusiform or saccular.7) A CA at an SES-implanted site accompanies stent malapposition. Although the precise mechanism of a CA at an SES-implanted site is unclear, several possible mechanisms, including acute vessel damage during coronary intervention, localized hypersensitivity to the polymer, and vascular remodeling as a result of high drug concentrations have been postulated.8) The CAG and coronary 64-MDCT findings in the present case suggested that the CA development may have been related to hypersensitivity to the SES at the contact sites between the vessel wall and the stent surface. Because we did not find a CA on the baseline CAG, the SES may have played an important role in CA development. In contrast, vascular inflammatory responses occurring in patients with CSS may not affect the development of a CA, because no elevated eosinophils were found on the complete blood count, and serum C-reactive protein was not elevated in this patient. Only one case of acute myocardial infarction has been reported due to plaque rupture in a patient with CSS.9) Furthermore, previous studies indicate that coronary artery involvement in patients with CSS is uncommon.10)
Sirolimus has an anti-inflammatory property that inhibits the local inflammatory response at the stent-implanted lesion site. Sirolimus may not only inhibit neointimal growth but also endothelial depletion at the stent-implanted site. Endothelial depletion caused by sirolimus may trigger the development of a CA secondary to smooth muscle cell degeneration.
The natural course and the appropriate treatment for a CA remain uncertain. However, it is certain that patients with a CA need prolonged aspirin and dual antiplatelet therapy. Surgical treatment is indicated for patients with a significant CA obstructive lesion or evidence of embolization leading to myocardial ischemia and progressive enlargement of the saccular type of CA leading to rupture. Because a CA at an SES-implanted coronary lesion may have a benign clinical course, it is very important to choose the optimal treatment plan carefully.
However, it has not been demonstrated that localized exaggerated hypersensitivity due to sirolimus, polymers, or stent metal triggers the development of a CA. The development of the CA in this patient seemed to be due to more complex pathogenesis than expected, so an indepth investigation would confirm the association between localized hypersensitivity vasculitis and incomplete endothelization.
Figures and Tables
Fig. 1
A: baseline cranial-view coronary angiography (CAG) demonstrated total occlusion of the mid left anterior descending (LAD) artery. B: the manual thrombus aspiration catheter is shown. C: restoration of LAD blood flow after thrombosuction. D and E: two overlapping sirolimus-eluting stents (SES) were implanted from the distal to the proximal LAD artery lesion after balloon dilation. F: final CAG shows the successfully implanted SESs, good thrombolysis, and myocardial infarction flow.
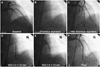
Fig. 2
A and B: a three-dimensional surface reconstruction and volume rendering image view of 64-multidetector computed tomography (MDCT) 14 months after primary percutaneous coronary intervention revealed good configuration of two overlapping sirolimus-eluting stents (SES) and no instent restenosis compared with the final coronary angiography (CAG). C: follow-up three-dimensional surface reconstruction view of coronary 64-MDCT at 39 months revealed diffuse enlargement of the left anterior descending (LAD) artery from the distal to the proximal stent border (triple white arrows). D: volume-rendering image shows crescent-like shaped contrast dye filling out of the stent border with a good configuration of the two overlapping SESs (triple white arrows). E: follow-up CAG shows diffuse multiple conglomeration contrast filling along the stent border without instent restenosis (triple black arrows).
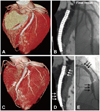
Fig. 3
Intravascular ultrasound assessment revealed a markedly enlarged left anterior descending (LAD) external elastic membrane (EEM) around the sirolimus-eluting stents (SES) struts. Maximal EEM size of the LAD was 6.59 mm at the proximal (p) portion, 6.42 mm at the mid (m) portion, and 6.82 mm at the distal (d) portion.
References
1. Windecker S, Remondino A, Eberli FR, et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med. 2005. 353:653–662.
2. Nienaber CA, Akin I, Schneider S, et al. Clinical outcomes after sirolimus-eluting, paclitaxel-eluting, and bare metal stents (from the first phase of the prospective multicenter German DES.DE registry). Am J Cardiol. 2009. 104:1362–1369.
3. Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007. 369:667–678.
4. Bavry AA, Chiu JH, Jefferson BK, et al. Development of coronary aneurysm after drug-eluting stent implantation. Ann Intern Med. 2007. 146:230–232.
5. Mavrogeni S, Manoussakis MN, Karagiorga TC, et al. Detection of coronary artery lesions and myocardial necrosis by magnetic resonance in systemic necrotizing vasculitides. Arthritis Rheum. 2009. 61:1121–1129.
6. Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990. 33:1094–1100.
7. Murthy PA, Mohammed TL, Read K, Gilkeson RC, White CS. MDCT of coronary artery aneurysms. AJR Am J Roentgenol. 2005. 184:3 Suppl. S19–S20.
8. Alfonso F, Pérez-Vizcayno MJ, Ruiz M, et al. Coronary aneurysms after drug-eluting stent implantation: clinical, angiographic, and intravascular ultrasound findings. J Am Coll Cardiol. 2009. 53:2053–2060.
9. Wagner AD, Meyer GP, Rihl M, et al. Acute coronary syndrome associated with Churg-Strauss syndrome. Vasc Health Risk Manag. 2007. 3:775–779.
10. Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet. 2003. 361:587–594.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download