Abstract
A 73-year-old woman with a history of chronic hypertension and severe chronic obstructive pulmonary disease, presented to a district general hospital with thoracic pain in a profound state of shock. She was diagnosed with cardiac tamponade, severe mitral regurgitation, and Stanford type A (Debakey type I) intramural hematoma. Her ascending aorta was of a significant size and therefore emergent repair was done to replace the ascending aorta and mitral valve. After 6 months, an increased aneurysmal size of 6.0 cm was observed in a follow up contrast-enhanced computed tomography angiography. The patient was successfully treated by a staged hybrid procedure involving initial supra-aortic reconstruction.
An aortic arch aneurysm is a well described pathological lesion of the thoracic aorta known to have a high risk of aortic rupture.1) Recently, a hybrid procedure combining surgical and endovascular repair has become established as a preferential treatment for extensive pathologies of the aorta.
We report a 73-year-old woman with an aortic arch aneurysm involving the distal aortic arch. We performed a combined procedure of extra-anatomic supra-aortic trunk de-branching and stent grafting of the aortic arch because of severe retrosternal adhesion and lung problems.
A 73-year-old woman with a history of chronic hypertension and severe chronic obstructive pulmonary disease (COPD), presented to a district general hospital with thoracic pain in a profound state of shock (blood pressure, 80/50 mmHg; pulse rate, 115 per minute; hemoglobin level, 8.7 g/dL). Echocardiogram and chest computed tomography (CT) scan showed a large pericardial effusion with right ventricular collapse which was consistent with cardiac tamponade (Fig. 1), as well as severe mitral regurgitation (MR). Closed pericardial drainage was immediately performed. Although over 500 mL of bloody fluid had been drained, the patient was stabilized with aggressive hydration and taken for an aorta CT scan to determine a dissection protocol. It revealed a Stanford type A (Debakey type I) intramural hematoma (IMH) extended into the distal portion of the left subclavian artery (SCA) with aneurysmal change. After the above-mentioned procedure, she was referred to our institution for further management.
The aortic arch between the innominate artery (IA) origin and the left SCA origin was preserved, and the maximum diameter was measured at 4.3 cm, which did not pose a significant risk for imminent rupture. However the IMH in the ascending aorta was significantly large and the risk of rupture was increasing. Therefore, emergent repair was planned.
Under circulatory arrest and deep hypothermia, the ascending aorta from the sinotubular junction to the proximal portion of the IA was resected and completely removed. Subsequently, a 30×16 mm InterGard graft® (Maquet, Rasttat, Germany) was used to replace the ascending aorta. In addition, mitral valve replacement was performed for MR.
After 6 month, an aorta CTA was performed, which showed that the aneurysmal size had increased from 4.3 to 6.0 cm, and the IA itself was aneurysmal (Fig. 2). A repeat of general anesthesia was difficult due to the patient's lung problems. The dissection of retrosternal adhesions may also be at risk for damage to the adjacent aneurysm, with a high possibility of rupture (Fig. 2).
Therefore, a staged hybrid procedure was performed with initial supra-aortic reconstruction because of the expected landing zone of the endograft covering all arch branch vessels. Magnetic resonance imaging (MRI) of distal carotid arteries and the vertebral arteries showed neither stenotic pathology nor anomalous origin from the aorta that may compromise cerebral blood flow during the procedure.
The first part of the hybrid procedure consisted of de-branching right-left SCA and right-left common carotid arteries (CCA). This was done via a low partial sternotomy without using cardiac bypass, where a 16×8 mm bifurcated trouser graft was anastomosed to the previous graft of the ascending aorta using a side biting arterial clamp. Two additional 8 mm grafts were sutured to each limb of the inverted trouser graft and all 4 limbs were then tunneled behind the clavicles. They were anastomosed end-to-end to both CCAs and end-to-side to the right and left SCA (Fig. 3). Though both CCAs were ligated to prevent type 2 endoleak, the VAs were maintained in circulation.
For the next stage, endovascular stent grafting was completed after 10 days. The left common femoral artery was punctured with a 6 Fr sheath and an aortogram showed the previous graft. A right common femoral access was prepared for inserting an Amplatz® vascular plug (AGA Medical Corp, Golden Valley, MN, USA). The 16 Fr sheath was inserted into the origin of the left SCA using a guidewire. A 16×12 mm Amplatz vascular plug II was then deployed into the left SCA through the sheath. The IA was occluded using the 16×12 mm Amplatz® vascular plug II in the same way.
The diameter of the graft was chosen with 20% over-sizing, and its length covered the aortic arch from the graft of the ascending aorta to the proximal SEAL thoracic stent graft (S&G Biotech INC., Seongnam, Korea) descending aorta. Consequently, a 38×150 mm stent graft was positioned using the guide wire to guarantee safe positioning. The landmark for stent graft placement was the origin of IA, and deployment was performed 3 cm proximally to this landmark. And, a second 40-38-100 mm tapered stent graft was overlapped to the first one to cover the full length of the aneurysm (Fig. 4). Follow up angiography showed good positioning and no leakage.
There were neither neurologic nor vascular complications. After one week, a post-procedural aorta CTA showed the complete exclusion of the aneurysm (Fig. 5), and the patient was discharged on the seventh post-procedural day in good general condition.
This report describes the feasibility of combined surgical and endovascular repair of extensive pathologies of the aorta. The hybrid procedure was designed for a patient with decreased pulmonary function experiencing difficulty to wean from the respirator and with retrosternal adhesion that could lead to a higher risk of rupture.
Without proper management a thoracic aortic aneurysm can worsen rapidly, with a rupture rate of approximately 50%.2-4) Although surgical repair technology has improved, it still carries a high mortality (10-16%) and is associated with severe morbidity.5-9) In addition, because of older age, as well as the frequency and severity of concomitant medical problems such as hypertension, COPD, cardiac disease, and chronic renal insufficiency, these patients are less than ideal candidates for conventional open surgery. Endovascular treatment may therefore play a considerable role in the treatment of these patients. The benefits of an endovascular approach include reduced mortality rates and reduced complication rates with specific reference to paraplegia and pulmonary complications.10)
However, there is a critical need for more complex endovascular techniques when the arch vessels are involved in the lesions leaving an insufficient proximal or distal landing zone. The technique has been developed to account for short landing zones and aneurysmal extension into the aortic arch segment by the use of hybrid de-branching techniques and branched stent grafts.
Recently there have been many reports of hybrid approaches to address thoracic arch aneurysms. Kang et al.11) reported the effectiveness and safety of employing the hybrid approach in patients with aortic arch pathologies, but the study is limited by its retrospective design, small sample size, diversity of thoracic aortic pathologies and a short follow-up duration. In an extensive meta-analysis to evaluate the overall results of the hybrid approach, Koullias12) showed that hybrid arch procedures are technically feasible in patients with a variety of arch diseases. Overall 30-day mortality was 8.3%, which is better than standard operative repair.
We selected a hybrid approach method to treat our patient because a full sternotomy had a higher risk of aortic rupture due to retrosternal adhesion. We delayed the stenting to a week after the de-brancing procedure to confirm maintained circulation in right-left CCAs and right-left SCA because the stenting has a possibility to block the flow of vessels. To the best of our knowledge, the current case is the first report of a complex thoracic aortic arch aneurysm with severe obstructive lung disease and retrosternal adhesion. Additionally, in cardiac tamponade due to arotic dissection, percutaneous pericardiocentesis can accelerate bleeding and shock, which is also observed in our patients.
In conclusion, our case shows a hybrid approach which was a combination of extra-anatomic supra-aortic trunk de-branching and stenting of the descending aorta. The approach may be a good strategy for the treatment of complex aortic pathology in patients who are less than ideal candidates for conventional open surgery.
Figures and Tables
Fig. 1
The aorta CTA at initial presentation. The CT scan showed a large pericardial effusion (A) and Stanford type A (Debakey type I) intramural hematoma (B). CTA: computed tomography angiography.
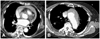
Fig. 2
The aorta CTA after 6 months. The CT scan showed an increase in aneurysmal size from 4.3 cm to 6.0 cm (A). It also showed retrosternal adhesions of arch aneurysm and manubrium of the sternum (B). The innominate artery itself was aneurysmal (C). CTA: computed tomography angiography.
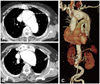
Fig. 3
The aorta CTA one week after the de-brancing procedure. The CT scan showed that the circulation was maintained in right-left CCAs and right-left SCA. CTA: computed tomography angiography, CCA: common carotid artery, SCA: subclavian artery.
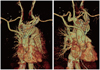
Fig. 4
Angiography during the stenting of the descending thoracic aorta. A 38×150 mm and 40-38-100 mm tapered SEAL thoracic stent graft (S&G Biotech INC., Seongnam, Korea) were deployed to cover the full length of the aneurysm.

Fig. 5
The aorta CTA one week after stenting of the descending thoracic aorta. The CT scan showed stent graft covering ascending aorta (A)-aortic arch (B)-proximal descending thoracic aorta. It also showed mural thrombus outside of the stent-graft. There was no evidence of contrast leakage from the anastomosis site of the graft and aortic arch (C). Bypass grafts from the ascending aorta to the bilateral CCA and SCA were patent (D). CTA: computed tomography angiography, CCA: common carotid artery, SCA: subclavian artery.
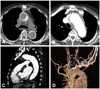
References
1. Alsac JM, Coscas R, Paraskevas N, Francis F, Castier Y, leseche G. Acute debranching and stent grafting for a ruptured penetrating ulcer of the aortic arch. Ann Vasc Surg. 2009. 23:687.e5–687.e8.
2. Pressler V, McNamara JJ. Thoracic aortic aneurysm: natural history and treatment. J Thorac Cardiovasc Surg. 1980. 79:489–498.
3. Perko MJ, Norgaard M, Herzog M, Olsen PS, Schröder TV, Pettersson G. Unoperated aortic aneurysms: a survey of 170 patients. Ann Thorac Surg. 1995. 59:1204–1209.
4. Pressler V, McNamara JJ. Aneurysms of the thoracic aorta. J Thorac Cardiovasc Surg. 1985. 89:50–54.
5. de Bakey ME, McCollum CH, Graham JM. Surgical treatment of aneurysms of the descending thoracic aorta: long-term results in 500 patients. J Cardiovasc Surg (Torino). 1978. 19:571–576.
6. Livesay JJ, Cooley DA, Ventemiglia RA, et al. Surgical experience in descending thoracic aneurysmectomy with and without adjuncts to avoid ischeamia. Ann Thorac Surg. 1985. 39:37–46.
7. Borst HG, Jurmann M, Bühner B, Laas J. Risk of replacement of descending aorta with a standardized left bypass technique. J Thorac Cardiovasc Surg. 1994. 107:126–133.
8. von Segesser LK, Killer I, Jenny R, Lutz U, Turina MI. Improved distal circulatory support for repair of descending thoracic aortic aneurysms. Ann Thorac Surg. 1993. 56:1373–1380.
9. Shim WH. Hybrid approach for complex thoracic aortic pathology. Korean Circ J. 2010. 40:368–369.
10. Sayed S, Thompson MM. Endovascular repair of the descending thoracic aorta: evidence for change in clinical practice. Vascular. 2005. 13:148–157.
11. Kang WC, Shin EK, Ahn TH, et al. Combined open endovascular repair for aortic arch pathology. Korean Circ J. 2010. 40:399–404.
12. Koullias GJ, Wheatley GH 3rd. State-of-the-art of hybrid procedures for the aortic arch: a meta-analysis. Ann Thorac Surg. 2010. 90:689–697.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download