Abstract
Background and Objectives
Local wide split double potentials are used as a parameter to determine complete conduction block during cavotricuspid isthmus ablation in patients with isthmus dependent atrial flutter. However, delayed slow conduction in that region can sometimes be very difficult to differentiate from complete block. Flutter cycle length (FCL) can be used to confirm isthmus conduction block, because FCL is a measure of conduction time around the tricuspid annulus (TA). This study was designed to determine which degree of splitting of the local electrograms is adequate to confirm complete isthmus block, using FCL as a reference.
Subjects and Methods
Cavotricuspid isthmus (CTI) ablation was performed in fifty consecutive patients. The interval between the pacing stimulus on the lateral side of the CTI and the first component of the double potentials on the block line (SD1) corresponded to the counterclockwise conduction time. The interval between the pacing stimulus and second component (SD2) represented the clockwise conduction time to the contralateral side of the ablation line. SD1 and SD2 were measured before and after complete isthmus block.
Conduction block of the cavotricuspid isthmus (CTI) is the endpoint of typical atrial flutter (AFL) ablation. Several methods for assessing that target have been suggested.1-6) Wide splitting of the local electrograms after creation of a line of block associated with unidirectional right atrial activation traveling in sequence along a multielectrode catheter is, in general, the method most widely used. However, in some cases, wide splitting of the local electrograms of greater than 110 ms, for example, may not be an indicator of complete block (Fig. 1), because the distance between double potentials is inversely proportional to the distance between the pacing electrode and first component of double potentials after achieving complete conduction block of the CTI. Further, it is not uncommon to see right atrial activation occurring in sequence, even with incomplete conduction block (Fig. 2), because the duodecapolar electrode catheter cannot cover the entire conduction path around the tricuspid valve annulus (TVA) when the right atrium is dilated, and sometimes it cannot be positioned anatomically parallel to the TVA. To date, there has been no single method that is not only very specific, but also simple and easily applicable to every case. Currently, a combination of several methods is needed in order to confirm complete block. On the other hand, the sum of the distances between the pacing site and each double potential recorded along the ablation line (SDSUM, SD1+SD2) may be equal to the activation time around the TVA when isthmus conduction is blocked (Fig. 3).
Therefore, the purpose of this study was to determine the usefulness of the comparison of SDSUM with flutter cycle length (FCL), which would be the shortest conduction time around the TVA in typical CTI-dependent flutter, in confirming complete conduction block of the CTI.
Subjects in this study consisted of 50 consecutive patients who underwent radiofrequency catheter ablation procedures for typical CTI-dependent flutter at the Chonbuk National University Hospital. There were 37 men and 13 women (mean age of 57±18, range of 16 to 84). Structural heart disease was present in 9 patients. This included valvular heart disease in 4 patients, Ebstein anomaly in 1 patient, and tachycardiomyopathy in 4 patients. Atrial flutter not induced by programmed electrical stimulation was excluded. Patients taking antiarrhythmic drugs were also excluded.
Informed consent was obtained from all patients. Via a femoral venous approach, a quadripolar electrode catheter (Atrial Fibrillation Division, St. Jude Medical, Minnetonka, MN, USA) was positioned on the lateral side of the CTI (CTI-L) for pacing in 19 patients. A 7 Fr duo-decapolar halo-type catheter (Livewire, Atrial Fibrillation Division, St. Jude Medical, Minnetonka, MN, USA) was positioned along the TVA instead of the quadripolar catheter in the remaining patients.7) A 3.5-mm ablation catheter with an irrigated tip (Thermocool, Biosense Webster, Johnson & Johnson, Diamond Bar, CA, USA) was used for mapping the isthmus and delivering radiofrequency energy. Electrograms were filtered at a bandpass filter setting of 30 to 500 Hz and recorded using a Prucka system (Prucka Engineering, Houston, TX, USA). Pacing was performed using a programmable stimulator (Bloom. Associates, Reading, PA, USA). CTI dependency of AFL was verified using the entrainment method and confirmation of full coverage of FCL when checking around the TVA.8)9) The radiofrequency linear ablation lesion started from the tricuspid side of the CTI at the 6 o'clock position in the left anterior oblique view during AFL and continued toward the inferior vena cava (IVC) during lower lateral right atrial pacing at a cycle length of 600 msec after termination of flutter. Radiofrequency (RF) energy was delivered along the CTI with individual 60-second applications, with a temperature limit of 50℃ and maximum power output of 40 W (IBI 1500T, Irvine Biomedical, Inc., Irvine, California, USA). After achieving complete conduction block of the CTI, reconfirmation was performed in all patients after 30 minutes. Reconduction occurred bidirectionally in 5 of 50 procedures while waiting. Repeat RF energy delivery was performed at the point showing the least widely split electrograms recorded along the line, and bidirectional block was once again achieved in all patients.
Complete clockwise (CW) and counterclockwise (CCW) CTI block were confirmed by analysis of the atrial activation sequence around the TVA during pacing from the septal side of the CTI (CTI-S) and CTI-L, respectively, and by the presence of widely split double potentials at all points along the ablation line.1) Complete bidirectional isthmus block was also confirmed in all patients according to the differential pacing method described by Shah et al.2) The interval between the pacing stimulus (S) at the CTI-L and first component of the split electrograms (D1) on the ablation line was defined as SD1. It represented CCW conduction time from the pacing site to the ablation site along the propagation course of the posterior aspect of the TVA. The interval between the pacing stimulus and second component was defined as SD2. This represented a prolonged conduction time due to slow conduction across the ablation line in the case of incomplete block, or the CW conduction time to the ablation line when conducting along the anterior aspect of the TVA when complete block was achieved. If there was no conduction through the line of block in the isthmus, SDSUM represented the entire conduction time around the TVA, as in Fig. 3. In CTI dependent flutter, FCL itself also represented the entire conduction time around the TVA. SDSUM was compared with FCL, as in Fig. 4. To accurately determine the longest possible conduction time through the ablation line during incomplete block, SD2 was also measured during application of radiofrequency energy immediately before a line of complete block was created and named SD2in. SD2in could not be obtained in 10 patients due to achievement of complete conduction block at the same time as flutter termination.
All variables were standardized proportionally to the mean value of the FCLs (224 ms) for easy comparison (Fig. 5) (Table 1). A Receiver Operating Characteristic curve was used to choose the best value (rule) for differentiation of incomplete and complete conduction block. Sensitivity, specificity, positive and negative predictive values, and false positive rate were calculated using the 90% rule (lower 90% of FCL) for testing completeness of the isthmus conduction block. When using an ROC curve, the 82% rule had the highest diagnostic accuracy (100% sensitivity), however, specificity was not 100%, and false positive rate was up to 2% (Table 2). Further, several of the SD1+SD2i values with incomplete conduction block were not far from the line of the 82% rule. Therefore, a 90% rule was used in this study to maintain 100% specificity and 0% false positive rate with adequate numerical difference from the sums of the incomplete conduction block, as well as for convenience.
Catheter ablation to achieve bidirectional CTI block was successful in all 50 procedures. Complete CW and CCW isthmus block were confirmed in each patient by three well known methods; activation sequence, widely split double potentials along the ablation line,1) and differential pacing, as described by Shah et al.2)
Twelve of the 50 AFLs were clockwise flutter. The interval between the pacing artifact and first and second components of the split potentials on the ablation line and the difference between FCL and SDSUM are shown in Table 1 and Fig. 5. SD1 was relatively stable during the ablation procedure, although it exhibited individual variation according to the distance between the pacing site and ablation line. SD2 exhibited progressive prolongation and a sudden wide jump as the ablation application progressed. As expected, SD2 was relatively shorter when SD1 was relatively longer after achieving complete conduction block (Fig. 3B). All patients whose SDSUM reached the FCL, or was very similar to the FCL, exhibited complete bidirectional conduction block. The period of incomplete isthmus block (SD2in) observed in 40 of the 50 procedures is shown in Table 1.
An SD1+SD2 reaching 90% of the FCL (90% rule) identified complete bidirectional isthmus conduction block with 94% sensitivity, 100% specificity, 100% positive predictive value, 93% negative predictive value, and 0% false positive rate (Table 2). Although our data showed complete bidirectional block after achieving the 90% rule in all cases, considering the theoretical background of our study, we aimed to confirm counterclockwise conduction block.
There have been several reports on how to confirm complete CTI conduction block.1-6) One included differential pacing, as described by Shah et al.2) Although it has been widely used, application is difficult in some patients. Proximal and distal electrode pairs should be parallel to the TVA and in good contact with the lower lateral right atrium for sequential pacing. This is not always easy because of the relationship between the IVC and lateral wall of the right atrium, particularly when performed by inexperienced personnel. The interval between the double potentials described by Tada el al.1) is prospective as data for isthmus conduction block. Even though it has a high degree of diagnostic accuracy, that interval partially depends on FCL, and mainly depends on the distance between the pacing site and first component of double potentials. If this distance is shorter, the interval between double potentials will be longer, and vice versa. As an extreme example, if pacing is performed just next to the completely blocked line, the first component of the double potentials would disappear and the second component would take almost the same time as the FCL to reach the other side of the ablation line, which would be much longer than 110 ms. Another reported method uses the transisthmus conduction interval described by Oral et al.3) Prolongation of the interval by >50% was suggested to represent complete conduction block, with a high degree of diagnostic accuracy. However, it was based on observational results and had the potential of including the borderline zone, although that was not significant in that study. Therefore, even though several methods have been reported, wide splitting of local double potentials on the ablation line and change in the activation sequence of a duodecapolar electrode catheter are the methods most widely used. However, these methods have pitfalls, as mentioned in the 'Introduction' of this report. Our method was as easy to perform as methods using the standard value (as 90-110 ms) of the interval between double potentials or the transisthmus interval, and, moreover, had a theoretical background to support the reason for complete CCW block.
The main concept of this study was that FCL is a measure of conduction time around the TVA in typical CTI dependent flutter. There are two ways to reach the line of block when pacing from the CTI-L. One way travels in the CCW direction (SD1), which takes a shorter time, and the other travels toward CW (SD2), which takes longer.
The sum of the distances covered by these two wavefronts is a complete circle around the TVA. Our hypothesis was that the sum of the conduction times in both the CW and CCW directions from the pacing site to the ablation line would be exactly the same as the FCL itself, unless the impulse is propagated across the ablation line. Further, this method could be used as a very simple and almost perfect indicator of complete CCW conduction block when it comes to typical CTI dependant flutter.
There were two observations that we did not notice at the beginning of the study. One was a conduction delay observed during a flutter that ran with a cycle length of about 250 ms. Ablation applications after termination of arrhythmia are usually continued during pacing at a pacing rate of 600 ms. A lesser degree of conduction delay would occur in this situation than during the faster cycle length of AFL. As the pacing rate increases, it takes longer to reach the ablation line from the pacing site, as in Fig. 6. That would be one of the most important factors preventing an exact matching of SDSUM with FCL. However, we focused on the similarity of SDSUM to its FCL as an indicator of the completeness of the line of block. We measured SD1 and SD2 just before and after wide splitting of the electrograms along the ablation line while pacing. When sums reached the lower 90% of the FCL, all cases exhibited complete isthmus conduction block that was bidirectional. Although there were SDSUMs that were 88% of the FCL (not reaching the 90% rule) in some cases of complete block, for convenience, we recommend use of the 90% rule.
This study had several limitations. First, comparing SDSUM with its FCL was assessed only in the counterclockwise direction. Unidirectional conduction was not observed in any cases using the 90% rule in the CCW direction since we checked the CW conduction block using other methods. If ablation was performed while pacing from the CTI-S from the beginning, or tested after repositioning of the duodecapolar catheter after ablation, we theoretically expected that it might also show the same results as SD1+SD2≅FCL in the clockwise direction. However, we did not use it to confirm the CW conduction block because repositioning the duodecapolar catheter for pacing while maintaining good widely split double potentials along the ablation catheter was more time consuming. Instead, we checked the atrial activation sequence of the distal pairs of electrodes on the duodecapolar catheter, which were positioned on the CTI-L around the TVA from the beginning of the procedure while pacing was performed using the ablation catheter, which was repositioned easily to the CTI-S position. We think that this may be the most convenient way to confirm CW block. Second, AFL turned around the TVA in one direction, but the pacing impulse was propagated in two directions. In the same way, conduction velocity could differ if the direction of propagation were reversed. However, there may not be such a large difference around the TVA, since we observed similarity in the cycle length of both the CW and CCW flutters. Third, if the impulse propagated across the ablation line too slowly, so that it reached the other side of the ablation line after creation of the second component of the double potentials via CW propagation, that of course would represent incomplete conduction block of the isthmus. However, as of yet, no ideal method of detection has been available. Besides, flutter recurrence would be difficult in that situation. Fourth, lower loop reentry around the IVC could not be completely excluded without use of a 3 dimensional mapping system. However, we confirmed full coverage of FCL when checking around the TVA using the duodecapolar electrode and ablation catheters.
Figures and Tables
Fig. 1
Displayed from the top, surface ECG leads I, aVF and V1, and intracardiac electrograms from a duodecapolar halo-type 19, 20 to 1, 2 from proximal to distal electrodes located in the right atrium around the tricuspid annulus, and ABLd: ablation catheter. A: wide splitting of the local double potentials (129 ms) is seen in the recording from ABLd, and the activation sequence from the halo-type catheter suggests counterclockwise conduction block of isthmus. B: while waiting following the ablation procedure, the activation sequence of the septal (proximal) electrodes on the halo-type catheter changed, suggesting re-conduction of the isthmus. Local double potentials became narrower, but still the interval between them was almost 110 ms, which is known as a specific indicator of complete isthmus block. C and D: left anterior oblique and right anterior oblique fluoroscopic view of the halo-type and ablation catheter. ECG: electrocardiogram.
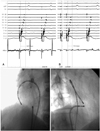
Fig. 2
Complete conduction block was achieved, and was associated with a sudden wide splitting of the local double potentials in the ABLd recording (the third beat). The activation sequence on the halo-type catheter suggests counterclockwise conduction block of the isthmus in all four paced beats, even before achievement of complete isthmus block. ABLd: ablation catheter.

Fig. 3
Activation times around the tricuspid annulus (TVA). A: flutter cycle length (FCL) is the activation time around the TVA. B: SD1: a shorter activation time between the lateral side of the cavotricuspid isthmus (CTI-L, pacing site) and line of conduction block (gray hatched). SD2: a longer activation time between the pacing site and line of block when the impulse turns anteriorly around the TVA. FCL=SD1+SD2.
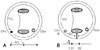
Fig. 4
Comparison between the flutter cycle length and the SDSUM. A: flutter cycle length (FCL) is 259 msec. B: conduction time taken from the stimulus (S) to the first potential (D1) of the split electrograms from ABLd, which corresponded to the counterclockwise distance from the lateral side of the cavotricuspid isthmus (CTI-L) to the ablation line in the isthmus, is 52 msec. Time from S to the second potential (D2), which corresponds to the clockwise conduction time from the CTI-L to the ablation line, when complete conduction block is achieved, is 196 msec. SDSUM is 248 ms (SD1+SD2 is nearly 95% of FCL). SDSUM: SD1+SD2, ABLd: ablation catheter.
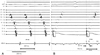
Fig. 5
Comparison of SDSUMs (SD1+SD2) to the mean flutter cycle length (FCL). All measured data are standardized proportionally to the mean value of the FCL (224 ms). The upper 50 dots (closed circles) show SD1+SD2s during complete isthmus block and the lower 40 dots (open circle) show that of traversing the ablation line just before the block. The upper and lower dots are a one to one match, except for the absence of some of the lower dots. Line 202 means 90% of the mean FCL.

Fig. 6
Conduction delay as the pacing cycle length being shorter. A: Recording during continuous pacing after making a complete line of conduction block in the cavotricuspid isthmus at a cycle length of 600 msec results in an usual pacing cycle length during flutter ablation, in which SD2 was 169 msec. B: SD2 becomes longer in reverse proportion as the pacing cycle becomes shorter due to a conduction delay. When the pacing cycle length reaches the flutter cycle length, as in B, split electrograms become smaller and are frequently indistinguishable. Fortunately, split electrograms become distinguishable in this case after increasing the local gain of the ABLd recording. ABLd: ablation catheter.

Table 1
Flutter cycle length and intervals between the stimulus and split potentials (msec)

SD1: interval between the stimulus and first component of the double potentials on the ablation line. SD2 and SD2in: intervals between the stimulus and second component of the double potentials after (in all cases) and just before (in 40 cases) achievement of complete conduction block. Each value represents mean±SD. FCL: flutter cycle length
Acknowledgments
This paper was supported by research funds from Chonbuk National University in 2004.
References
1. Tada H, Oral H, Sticherling C, et al. Double potentials along the ablation line as a guide to radiofrequency ablation of typical atrial flutter. J Am Coll Cardiol. 2001. 38:750–755.
2. Shah D, Haissaguerre M, Takahashi A, Jais P, Hocini M, Clementy J. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000. 102:1517–1522.
3. Oral H, Sticherling C, Tada H, et al. Role of transisthmus conduction intervals in predicting bidirectional block after ablation of typical atrial flutter. J Cardiovasc Electrophysiol. 2001. 12:169–174.
4. Matsushita T, Chun S, Liem LB, Friday KJ, Sung RJ. Unidirectional conduction block at cavotricuspid isthmus created by radiofrequency catheter ablation in patients with typical atrial flutter. J Cardiovasc Electrophysiol. 2002. 13:1098–1102.
5. Cosío FG, Awamleh P, Pastor A, Núñez A. Determining inferior vena cava-tricuspid isthmus block after typical atrial flutter ablation. Heart Rhythm. 2005. 2:328–332.
6. Kim JJ, Kim YH, Chung SS, et al. Radiofrequency catheter ablation in patients with atrial flutter. Korean Circ J. 1996. 26:605–613.
7. Saoudi N, Ricard P, Rinaldi JP, Yaïci K, Darmon JP, Anselme F. Methods to determine bidirectional block of the cavotricuspid isthmus in radiofrequency ablation of typical atrial flutter. J Cardiovasc Electrophysiol. 2005. 16:801–803.
8. Mangat I, Tschopp DR Jr, Yang Y, Cheng J, Keung EC, Scheinman MM. Optimizing the detection of bidirectional block across the flutter isthmus for patients with typical isthmus-dependent atrial flutter. Am J Cardiol. 2003. 91:559–564.
9. Deisenhofer I, Estner H, Zrenner B, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace. 2006. 8:573–582.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download