Abstract
Purpose
To investigate the pharmacologic modulation of motor task-dependent physiologic responses by antiplatelet agent, clopidogrel, during hand motor tasks in healthy subjects.
Materials and Methods
Ten healthy, right-handed subjects underwent three functional magnetic resonance (fMRI) sessions: one before drug administration, one after high dose drug administration and one after reaching drug steady state. For the motor task fMRI, finger flexion-extension movements were performed. Blood oxygenation level dependent (BOLD) contrast was collected for each subject using a 3.0 T VHi (GE Healthcare, Milwaukee, USA) scanner. T2*-weighted echo planar imaging was used for fMRI acquisition. The fMRI data processing and statistical analyses were carried out using SPM2.
Results
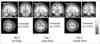
Second-level analysis revealed significant increases in the extent of activation in the contralateral motor cortex including primary motor area (M1) after drug administration. The number of activated voxels in motor cortex was 173 without drug administration and the number increased to 1049 for high dose condition and 673 for steady-state condition respectively. However, there was no significant difference in the magnitude of BOLD signal change in terms of peak T value.
Figures and Tables
References
1. Kimberg D, Aguirre G, Lease J, D'Esposito M. Cortical effects of bromocriptine, a D-2 dopamine receptor agonist, in human subjects, revealed by fMRI. Hum Brain Mapp. 2001. 12:246–257.
2. Honey G, Bullmore E, Soni W, Varatheesan M, Williams S, Sharma T. Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci USA. 1999. 96:13432–13437.
3. Braus D, Ende G, Weber-Fahr W, et al. Antipsychotic drug effects on motor activation measured by functional magnetic resonance imaging in schizophrenic patients. Schizophr Res. 1999. 39:19–29.
4. Loubinoux I, Boulanouar K, Ranjeva J-P, et al. Cerebral functional magnetic resonance imaging activation modulated by a single dose of the monoamine neurotransmission enhancers fluoxetine and fenozolone during hand motor tasks. J Cereb Blood Flow Metab. 1999. 19:1365–1375.
5. Goldstein L, Coviello A, Miller G, Davis J. Norepinephrine depletion impairs motor recovery following sensorimotor cortex injury in the rat. Restor Neurol Neurosci. 1991. 3:41–47.
6. Goldstein L. Common drugs may influence motor recovery after stroke. Neurology. 1995. 45:865–871.
7. Gubitz G, Sandercock P, Counsell C. Antiplatelet therapy for acute ischemic stroke. The Cochrane Library. 2000. (Issue 1):Oxford:
8. Tokumaru S, Yoshikai T, Uchino A, Matsui M, Kuroda Y, Kudo S. Technetium-99m-ECD SPECT in antiphospholipid antibody syndrome: a drastic improvement in brain perfusion by antiplatelet therapy. Eur Radiol. 2001. 11:2611–2615.
9. Chan F, Ching J, Hung L, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. New Engl J Med. 2005. 352:238–244.
10. Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med. 2003. 3:113–122.
11. Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric paps in functional imaging: a general linear approach. Hum Brain Mapp. 1995. 2:189–210.
12. Evans A, Collins D, Mills S, Brown E, Kelly R, Peters T. 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE-Nucl Sci Symp Med Imaging. 1993. 1813–1817.
13. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 1988. New York: Thieme.
14. Hochberg Y, Tamhane A. Multiple comparisons procedures. 1987. New York: Wiley.
15. Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A. A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Mapp. 1996. 4:58–73.
16. Mintzer M, Griffiths RR. Acute dose-effects of scopolamine on false recognition. Psychopharmacology. 2001. 153:425–433.
17. Bozzali M, MacPherson SE, Dolan RJ, Shallice T. Left prefrontal cortex control of novel occurrences during recollection: a psychopharmacological study using scopolamine and event-related fMRI. Neuroimage. 2006. 33:286–295.
18. Hasbroucq T, Rihet P, Blin O, Possamai C. Serotonin and human information processing: fluvoxamine can improve reaction time performance. Neurosci Lett. 1997. 229:204–208.
19. Weisskoff R, Zuo C, Boxerman J, Rosen B. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med. 1994. 31:601–610.
20. Lee S-P, Silva A, Ugurbil K, Kim S-G. Diffusion-weighted spin-echo fMRI at 9.4T: microvascular/tissue contribution to BOLD signal change. Magn Reson Med. 1999. 42:919–928.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download