Abstract
Metachromatic leukodystrophy (MLD; MIM 250100), a severe neurodegenerative disorder inherited as an autosomal recessive trait, is caused by mutations in the arylsulfatase A (ARSA) gene. Although several germ line ARSA mutations have been identified in patients with MLD of various ethnic backgrounds elsewhere in the world, no genetically confirmed cases of MLD have been reported in Korea. Recently, we identified a mutation in the ARSA gene of a Korean male with MLD. A male infant with late-infantile form of MLD had been admitted to our hospital for further examination. His neuromuscular symptoms, which included inability to walk at the age of 12 months, gradually worsened, even after allograft bone marrow transplantation; he died at the age of 9 yr. His elder brother had also been diagnosed with MLD. To confirm the presence of a genetic abnormality, all the coding exons of the ARSA gene and the flanking introns were amplified by PCR. A molecular analysis of the ARSA gene revealed both a novel heterozygous splicing mutation (c.1101+1G>T) in intron 6 and a heterozygous missense mutation in exon 2 (c.296G>A; Gly99Asp). The patient's elder brother who had MLD is believed to have had the same mutation, which may be correlated with a rapidly deteriorating clinical course. This study identified a novel mutation in the ARSA gene, related to a late-infantile form of MLD with a lethal clinical course and suggested that molecular diagnosis of patients may be useful in early diagnosis and for deciding intervention measures for their family members.
REFERENCES
1.Ługowska A., Szymańska K., Kmiec T., Tarczyńska I., Czartoryska B., Tylki-Szymańska A, et al. Homozygote for mutation c.1204+1G>A of the ARSA gene presents with a late-infantile form of metachromatic leukodystrophy and a rare MRI white matter lesion type. J Appl Genet. 2005. 46:337–9.
2.Coulter-Mackie MB., Gagnier L., Beis MJ., Applegarth DA., Cole DE., Gordon K, et al. Metachromatic leucodystrophy in three families from Nova Scotia, Canada: a recurring mutation in the arylsulphatase A gene. J Med Genet. 1997. 34:493–8.

3.Zlotogora J., Furman-Shaharabani Y., Harris A., Barth ML., von Figura K., Gieselmann V. A single origin for the most frequent mutation causing late infantile metachromatic leucodystrophy. J Med Genet. 1994. 31:672–4.

4.Gieselmann V. Metachromatic leukodystrophy: genetics, pathogenesis and therapeutic options. Acta Paediatr Suppl. 2008. 97:15–21.

5.Barth ML., Fensom A., Harris A. The arylsulphatase A gene and molecular genetics of metachromatic leucodystrophy. J Med Genet. 1994. 31:663–6.

6.Roh MR., Lee KW., Lee DW., Lee SJ., Lee DW., Kang DY, et al. Two cases of late infantile metachromatic leukodystrophy. J Korean Pediatr Soc. 1984. 27:1033–9.
7.Lee HJ., Shin YJ., Hwang YS., Moon HR., Seo JS. Late infantile metachromatic leukodystrophy-arylsulfatase A assay in 24h urine. J Korean Pediatr Soc. 1989. 32:978–83.
8.Kim YJ., Chae KY., Choi JE., Kim KJ., Hwang YS., Kim IO. Clinical and neurologic features of metachromatic leukodystrophy. J Korean Child Neurol Soc. 1995. 3:31–42.
9.Kreysing J., von Figura K., Gieselmann V. Structure of the arylsulfatase A gene. Eur J Biochem. 1990. 191:627–31.

10.Rogan PK., Faux BM., Schneider TD. Information analysis of human splice site mutations. Hum Mutat. 1998. 12:153–71.

11.Kondo R., Wakamatsu N., Yoshino H., Fukuhara N., Miyatake T., Tsuji S. Identification of a mutation in the arylsulfatase A gene of a patient with adult-type metachromatic leukodystrophy. Am J Hum Genet. 1991. 48:971–8.
12.Polten A., Fluharty AL., Fluharty CB., Kappler J., von Figura K., Gieselmann V. Molecular basis of different forms of metachromatic leukodystrophy. N Engl J Med. 1991. 324:18–22.

13.Conzelmann E., Sandhoff K. Partial enzyme deficiencies: residual activities and the development of neurological disorders. Dev Neurosci. 1983. 6:58–71.

14.Fluharty AL. Arylsulfatase A deficiency. Pagon RA, Bird TC, editors. GeneReviews;http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=mld[ME4. ] (Updated on Sep. 2008.
Fig. 1.
Brain magnetic resonance image. Axial T2-weighted image shows bilateral, diffuse confluent hyperintensities in the periventricular white matter and cerebral atrophy.
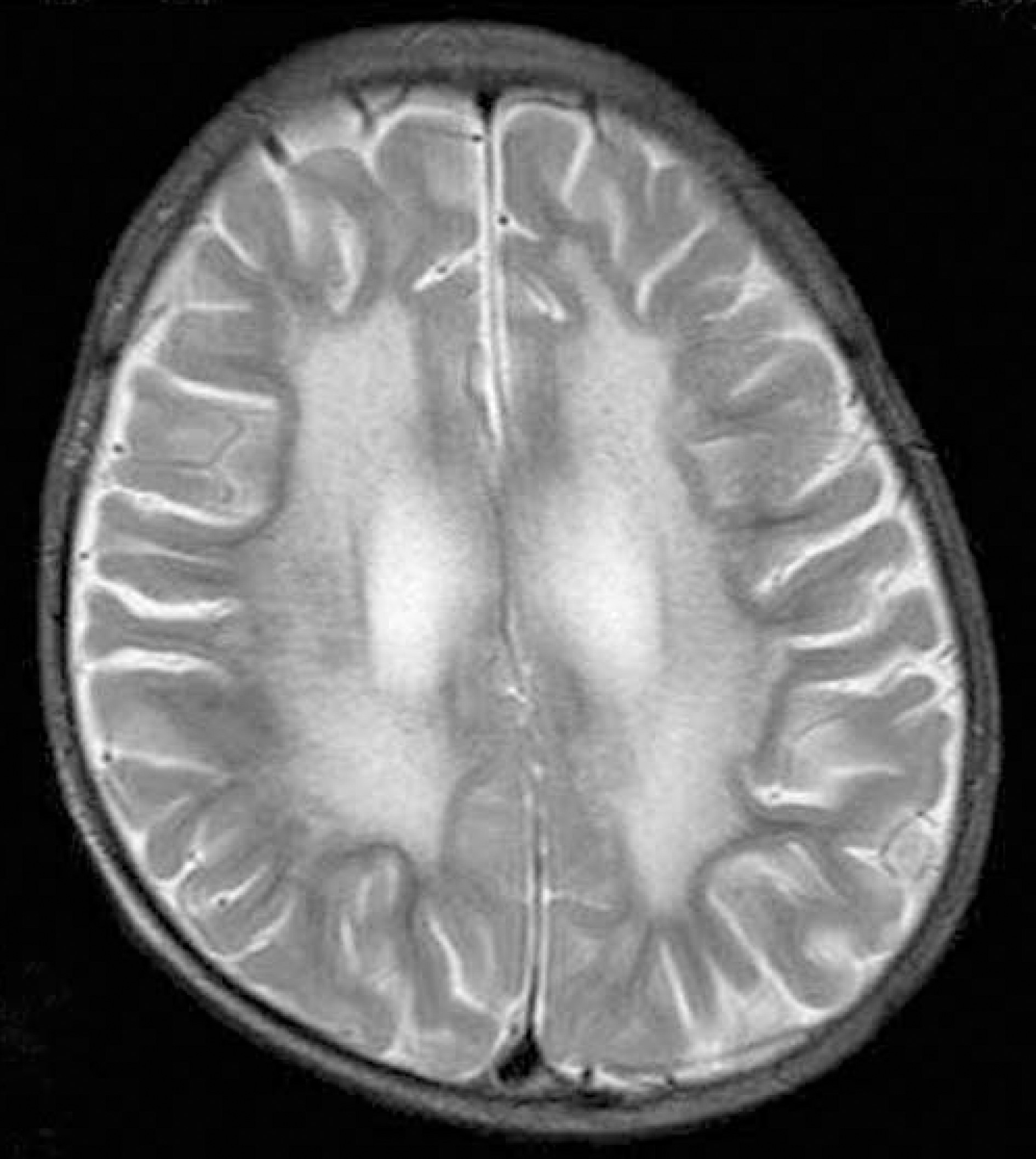
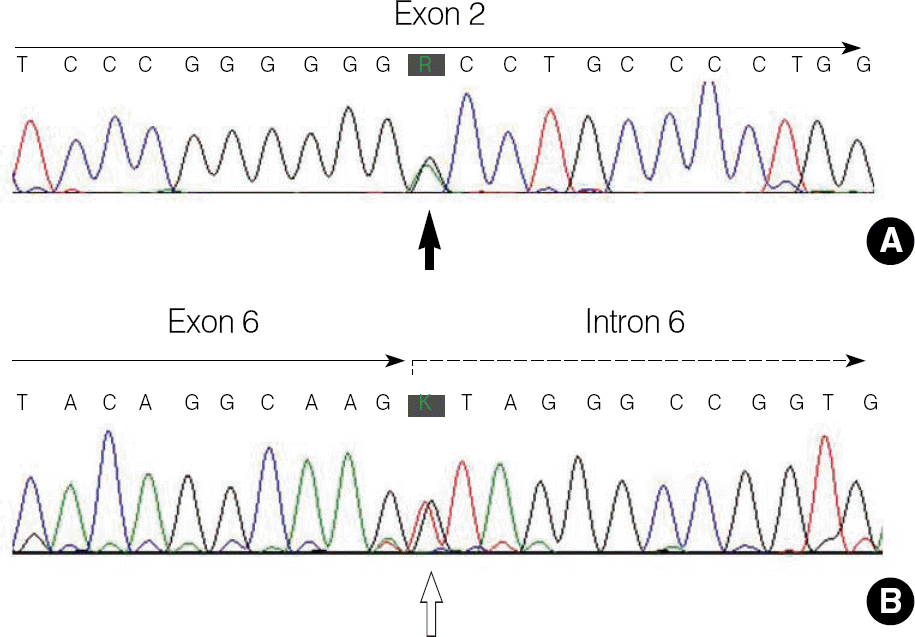
Fig. 2.
Mutation analysis of the arylsulfatase A (ARSA) gene in a Korean patient with metachromatic leukodystrophy. Direct sequencing of the ARSA gene shows overlapped peaks (solid arrow) at the nucleotide position 296 due to a heterozygous G-to-A transition (c.296G>A; Gly99Asp) (A) and overlapped peaks (open arrow) at the consensus splicing donor site in the intron 6 due to a heterozygous G-to-T transversion (c.1101+1G>T) (B).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download