Abstract
Purpose
To determine whether early visual acuity response to intravitreal bevacizumab in macular edema (ME) secondary to branch retinal vein occlusion (BRVO) is associated with 12-month follow-up outcome.
Methods
Sixty treatment-naïve patients (60 eyes) with ME secondary to BRVO treated with intravitreal bevacizumab (1.25 mg) were retrospectively included. Initially, all patients were injected monthly to achieve fluid resolution and followed up with a pro re nata regimen for at least 12 months. The relationship between early (month 1) and late (month 12) visual acuity response (mean change from baseline in best-corrected visual acuity [BCVA]; categorized improvement [<1, 1‒3, or ≥3 logMAR lines in BCVA]) was explored.
Results
The proportions of eyes with <1, 1-<3, and ≥3-line improvements at 1 month were 19 eyes (31.7%), 17 eyes (28.3%), and 24 eyes (40%), respectively. Within each of the three response categories, the mean BCVA change from baseline at 12 months and onward did not vary by more than 1 line from the observed mean BCVA improvement at 1 month. Inter-cohort differ-ences across the three response categories in mean BCVA change from baseline were statistically significant at each time point. Early BCVA response at 1 month showed significant associations with ≥3 line improvement and BCVA response at 12 months in multiple logistic and linear regression analyses.
References
1. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch ophthalmol. 2008; 126:513–8.
2. Rogers S, McIntosh RL, Cheung N. . The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010; 117:313–9.e1.

3. Mitchell P, Smith W, Chang A. Prevalence and associations of reti-nal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996; 114:1243–7.

5. Eye Disease Case-Control Study Group. Risk factors for branch retinal vein occlusion. Am J Ophthalmol. 1993; 116:286–96.
6. Sperduto RD, Hiller R, Chew E. . Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: the eye disease case-control study. Ophthalmology. 1998; 105:765–71.
7. The Branch Vein Occlusion Study Group. Argon laser photo-coagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984; 98:271–82.
8. Shilling JS, Jones CA. Retinal branch vein occlusion: a study of ar-gon laser photocoagulation in the treatment of macular oedema. Br J Ophthalmol. 1984; 68:196–8.

9. Noh GM, Lee JE, Nasm KY. . Macular ischemia correlated with final visual outcome in retinal vein occlusion patients. J Korean Ophthalmol Soc. 2014; 55:1493–8.

10. Rogers SL, McIntosh RL, Lim L. . Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1094–101.e5..

11. Chew EY, Ferris III FL. Nonproliferative diabetic retinopathy. Ryan SJ, Schachat AP, Hinton DR, Wilkinson CP, editors. Retina, 4th ed. St Louis: Mosby Elsevier Inc.;2006. v. 2.:p. chap. 67.

12. Browning DJ. Retinal Vein Occlusions: Evidence-based Management. Springer Science & Business Media. 2012; 265–78.
13. Gonzalez VH, Campbell J, Holekamp NM. . Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol. 2016; 172:72–9.

14. Chung EJ, Hong YT, Lee SC. . Prognostic factors for visual outcome after intravitreal bevacizumab for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1241–7.

15. Miwa Y, Muraoka Y, Osaka R. . Ranibizumab for macular ede-ma after branch retinal vein occlusion: One initial injection versus three monthly injections. Retina. 2017; 37:702–9.
16. Kang JW, Lee H, Chung H, Kim HC. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after intravitreal bevacizumab for macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2014; 252:1413–21.

17. Park B, Kim J, Chung H, Kim HC. Correlation of fundus auto-fluorescence with foveal microstructures and vision in branch reti-nal vein occlusion. Retina. 2014; 34:531–8.

18. Kang KT, Kim YC, Kim KS. Factors related to repeatability of in-travitreal bevacizumab injections in branch retinal vein occlusion macular edema. J Korean Ophthalmol Soc. 2015; 56:1580–5.

19. Baek SU, Kwon SI, Park IW, Choi KJ. Consecutive macular edema and visual outcome in branch retinal vein occlusion. J Ophthalmol. 2014; 2014:439483.

20. Clark WL, Boyer DS, Heier JS. . Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-weekresults of the VIBRANT study. Ophthalmology. 2016; 123:330–6.
21. Brown DM, Campochiaro PA, Bhisitkul RB. . Sustained bene-fits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011; 118:1594–602.

22. Narayanan R, Panchal B, Stewart MW. . Grid laser with modi-fied pro re nata injection of bevacizumab and ranibizumab in mac-ular edema due to branch retinal vein occlusion: MARVEL report no 2. Clin Ophthalmol. 2016; 10:1023–9.

23. El‐ Shazly SF, El‐ Bradey MH, Tameesh MK. Vascular endothelial growth factor gene polymorphism prevalence in patients with dia-betic macular oedema and its correlation with anti‐ vascular endo-thelial growth factor treatment outcomes. Clin Exp Ophthalmol. 2014; 42:369–78.
24. Kloeckener-Gruissem B, Barthelmes D, Labs S. . Genetic as-sociation with response to intravitreal ranibizumab in patients with neovascular AMD. Invest Ophthalmol Vis Sci. 2011; 52:4694–702.

25. Kaneda S, Miyazaki D, Sasaki S. . Multivariate analyses of in-flammatory cytokines in eyes with branch retinal vein occlusion: relationships to bevacizumab treatment. Invest Ophthalmol Vis Sci. 2011; 52:2982–8.

26. Kang JW, Yoo R, Jo YH, Kim HC. Correlation of microvascular structures on optical coherence tomography angiography with vis-ual acuity in retinal vein occlusion. Retina. 2016; Nov 8:[Epub ahead of print].

27. Lim HB, Kim MS, Jo YJ, Kim JY. Prediction of retinal ischemia in branch retinal vein occlusion: Spectral-domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2015; 56:6622–9.

28. Kang HM, Chung EJ, Kim YM, Koh HJ. Spectral-domain optical coherence tomography (SD-OCT) patterns and response to intra-vitreal bevacizumab therapy in macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2013; 251:501–8.

Figure 1.
Mean best-corrected visual acuity (BCVA) changes from baseline over time, categorized by BCVA response at 1 month after the first bevacizumab injection. Within the groups, the mean BCVA changes from baseline did not vary by more than 1 line af-ter 1 month over the entire period. Inter-co-hort differences were significant at each time point. Post-hoc test (Tukey method) between group comparison. <1 line im-provement vs. ≥3 lines Improvement, p < 0.001, at each time point. 1-<3 line im-provement vs. ≥3 lines Improvement, p < 0.001, p = 0.016, p = 0.022, p = 0.011.
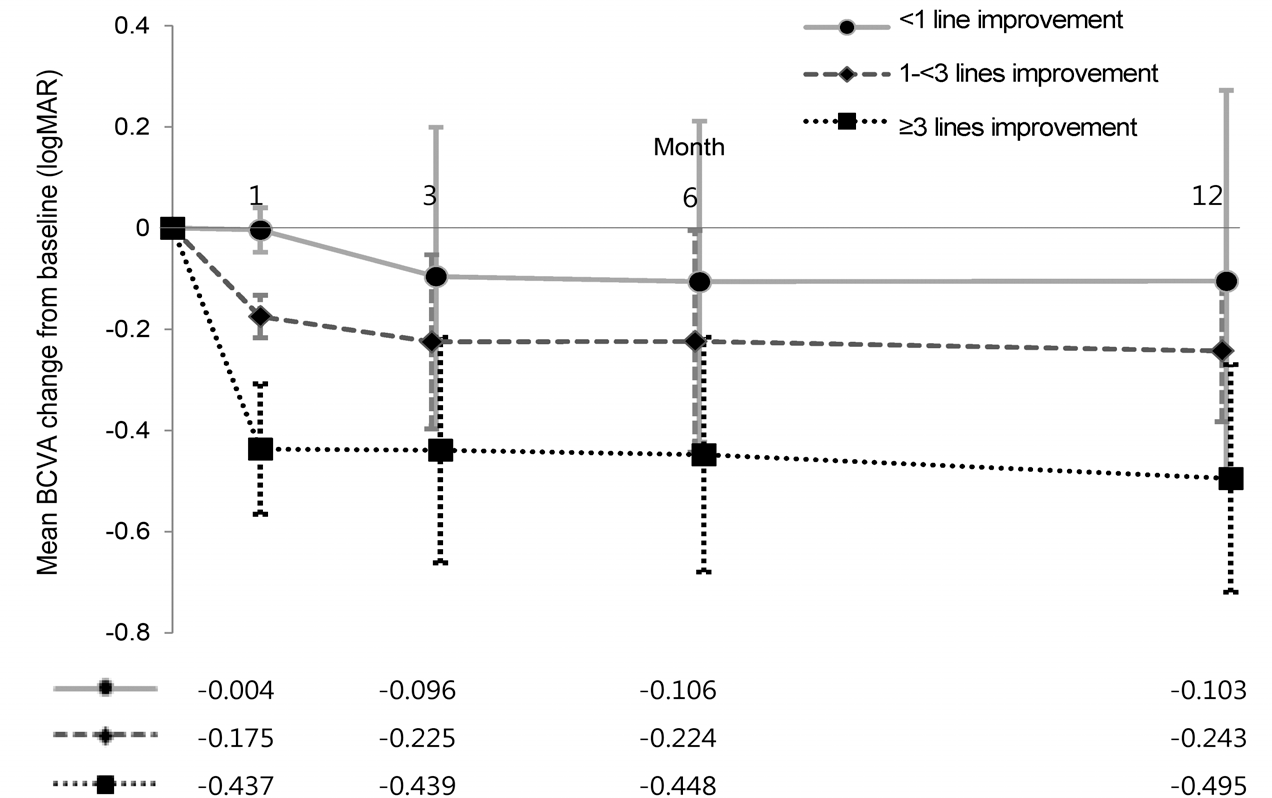
Figure 2.
Frequency distribution of best-corrected visual acuity (BCVA) improvement, categorized by BCVA response at 1 month. (A) Within the subset of eyes with < 1 line BCVA improvement at 1 month, approx-imately one-third of eyes continued to show < 1 line BCVA improve-ment at each time point. (B) Within the subset of eyes with 1 -< 3 lines BCVA improvement at 1 month, most eyes showed ≥ 1 line BCVA improvement at each time point. (C) Within the subset of eyes with a pro-nounced initial BCVA response (≥ 3 lines improvement) at 1 month, most eyes maintained this response over the entire period.
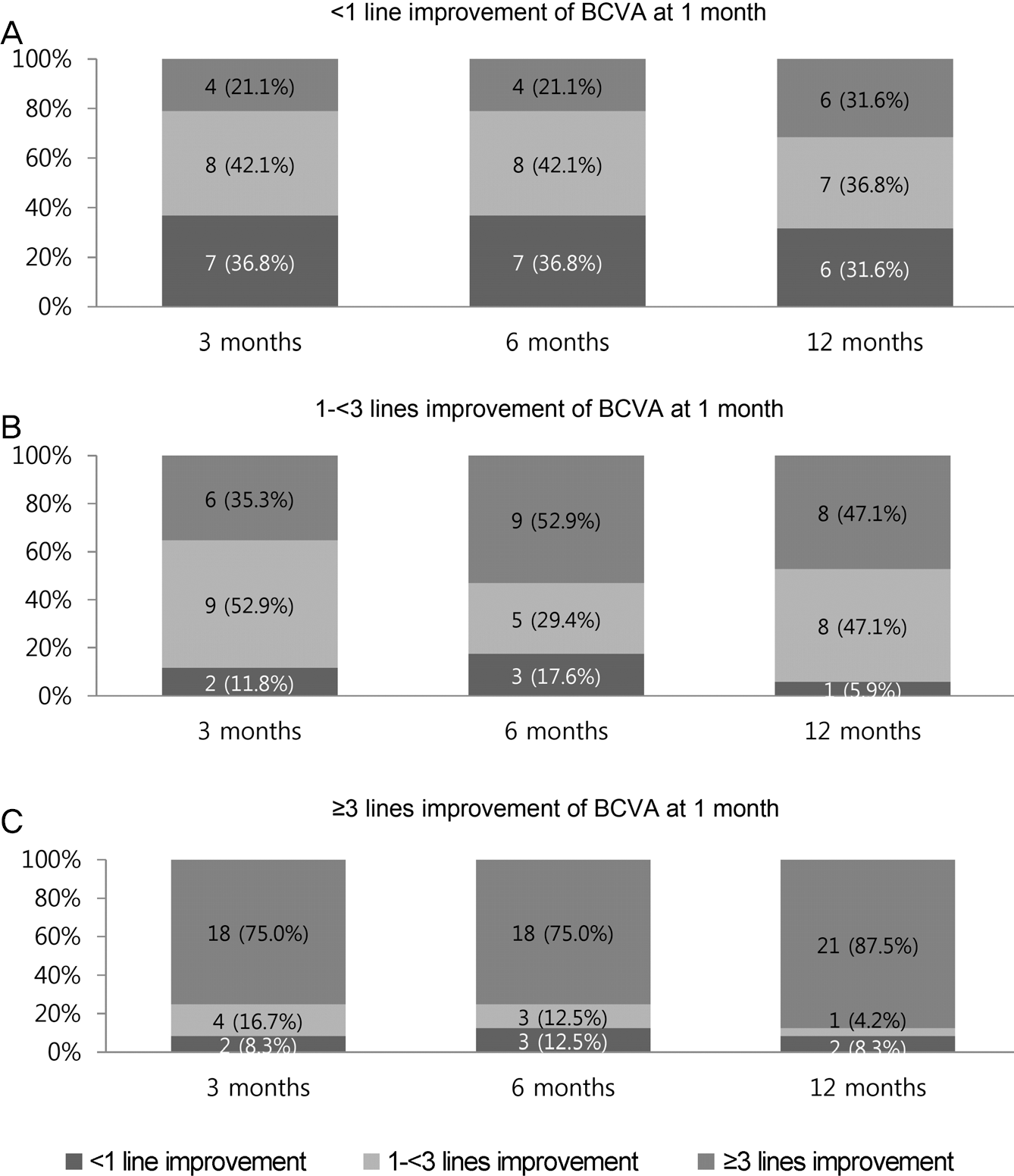
Figure 3.
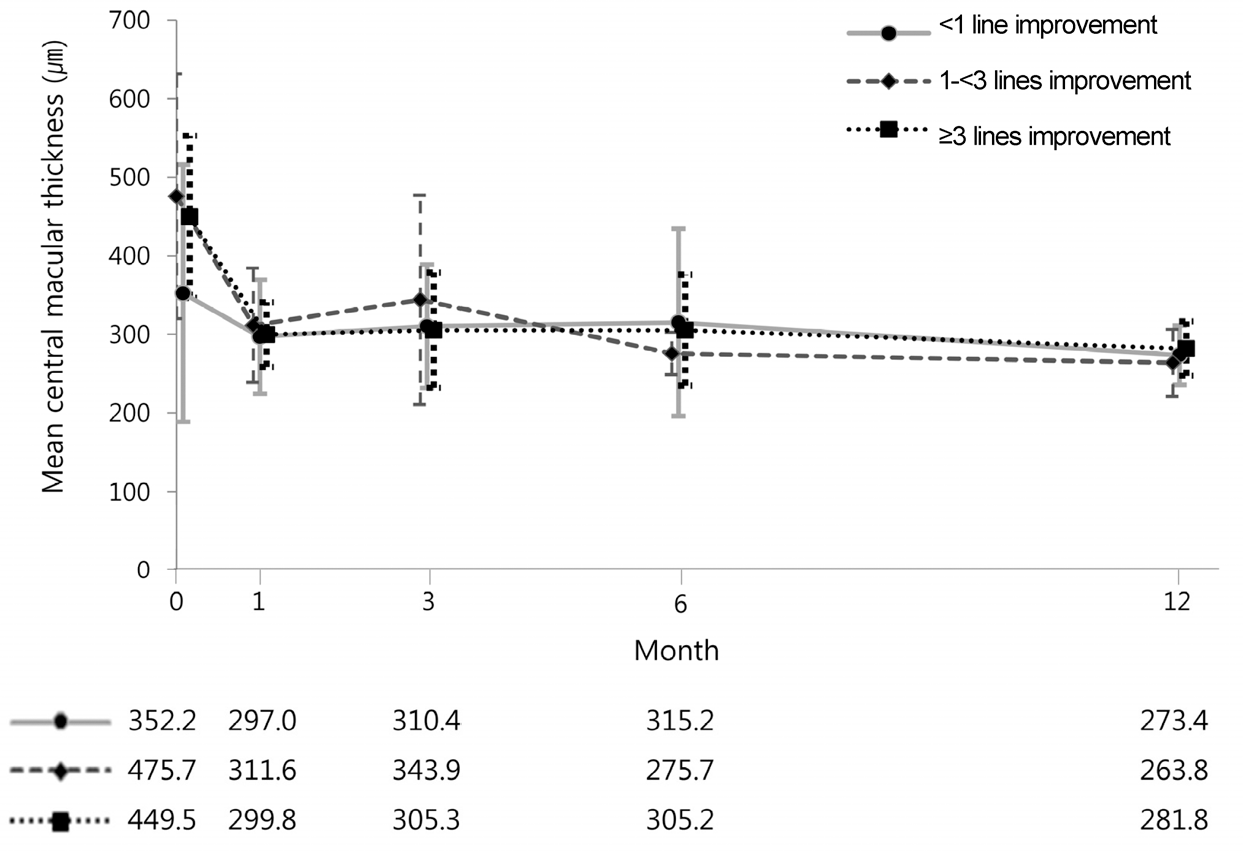
Mean central macular thickness (CMT) over time, categorized by best-cor-rected visual acuity response at 1 month after the first bevacizumab injection. There were no inter-cohort differences in mean CMT at each time point (p = 0.061, p = 0.651, p = 0.600, p = 0.420, p = 0.353, at each time point).
Supplementary Figure 1.
Structural changes of external limiting membrane (ELM) and ellipsoid zone (EZ) integrity over time, cate-gorized by best-corrected visual acuity (BCVA) response at 1 month. No significant difference in distribution of ELM and EZ re-storation amongh three groups at each time point. (A) ELM integrity over time in <1 line BCVA improvement group. (B) EZ in-tegrity over time in <1 line BCVA improvement group. (C) ELM integrity over time in 1-< 3 lines BCVA improvement group. (D) EZ integrity over time in 1-< 3 lines BCVA improvement group. (E) ELM integrity over time in ≥3 lines BCVA improvement group. (F) EZ integrity over time in ≥3 lines BCVA improvement group.
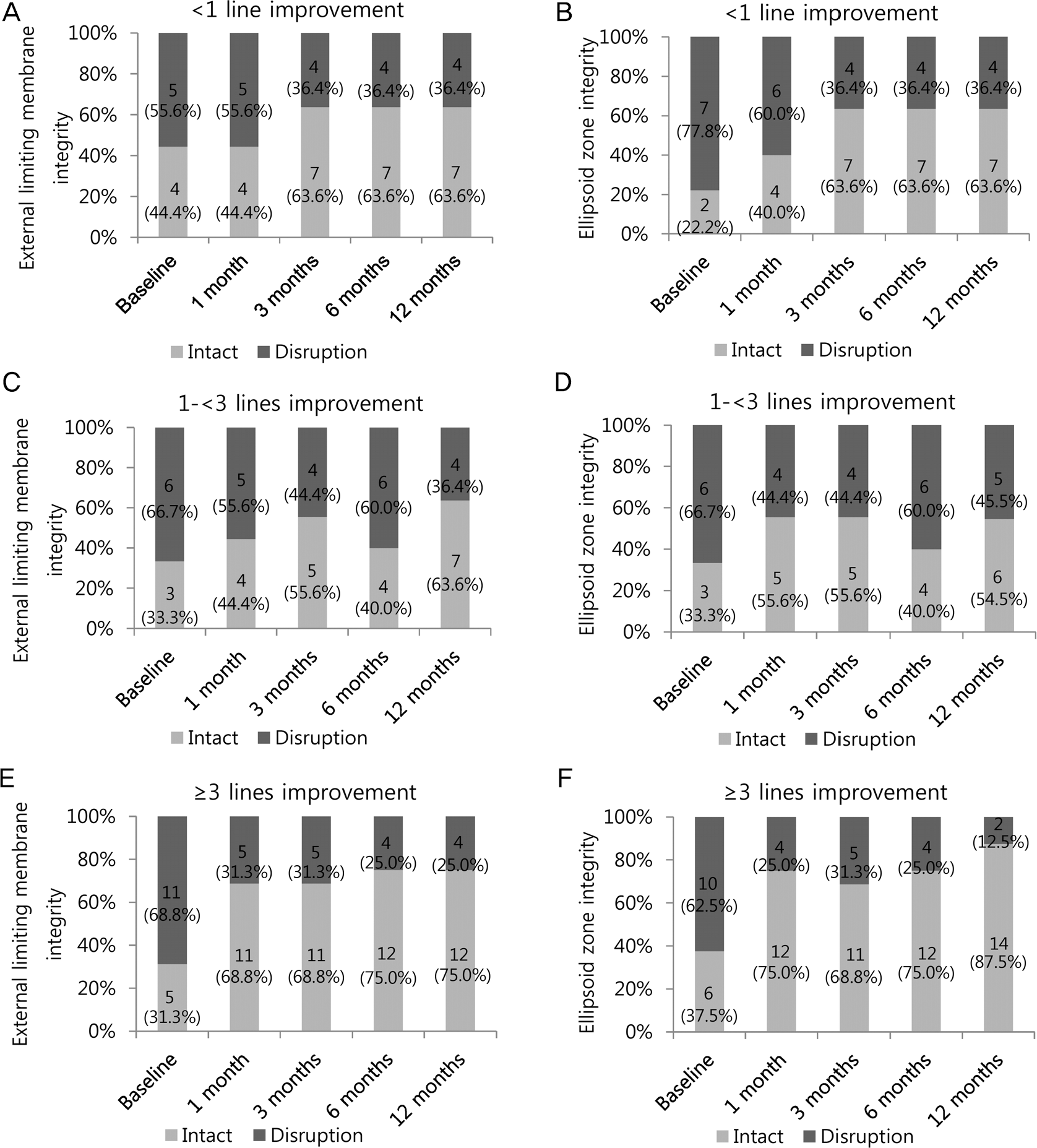
Table 1.
Demographic and baseline characteristics of pooled study eyes, categorized by BCVA response at 1 month after the first bevacizumab injection
| BCVA changes from baseline at 1 month | ||||
|---|---|---|---|---|
| <1 line improvement | 1-<3 lines improvement | ≥3 lines improvement | p-value | |
| (n = 19) | (n = 17) | (n = 24) | ||
| Age (years) | 62.3 ± 8.8 | 60.8 ± 10.7 | 61.8 ± 11.1 | 0.900* |
| Male: Female | 8:11 | 7:10 | 13:11 | 0.640† |
| Hypertension | 6 | 7 | 8 | 0.877† |
| Lens status (phakic:pseudophakic) | 16:3 | 12:5 | 22:2 | 0.202† |
| Symptom duration (months) | 3.8 ± 2.1 | 4.3 ± 2.9 | 1.6 ± 0.4 | 0.872‡ |
| Baseline BCVA (logMAR) | 0.45 ± 0.05 | 0.52 ± 0.07 | 0.68 ± 0.06 | 0.004‡§ |
| Baseline CMT (μ m) | 352.2 ± 47.3 | 475.7 ± 44.9 | 449.5 ± 25.0 | 0.061‡ |
| Foveal ischemia | 1 | 4 | 3 | 0.274† |
| Hyper-reflective foci | ||||
| Intra-retinal | 3.6 ± 4.9 | 2.9 ± 5.0 | 1.1 ± 1.6 | 0.259* |
| Outer-retinal | 2.6 ± 3.6 | 4.2 ± 5.5 | 2.3 ± 3.0 | 0.477* |
| Sub-retinal | 0.2 ± 0.7 | 1.1 ± 2.0 | 0.3 ± 0.9 | 0.226* |
Table 2.
Logistic regression analysis of 3 lines or more visual improvement at 12 months
Table 3.
Linear regression analysis of change from baseline in BCVA at 12 months




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download