Abstract
Purpose
To compare the efficacy of an intralesional steroid injection in the treatment of chalazion according to triamcinolone acetonide (TA) concentrations.
Methods
A total of 108 patients with 120 chalazia received an intralesional injection of TA. Patients were divided into 3 groups according to the concentrations of TA: 5 mg/ml, 10 mg/ml, and 40 mg/ml. A regular follow-up was performed and the size of lesion and recurrence were evaluated.
Results
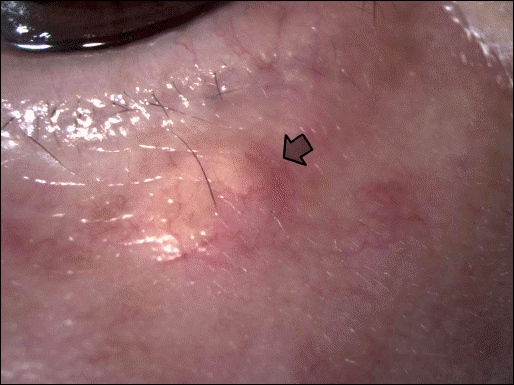
Success was defined as a minimum of 80% decrease in size with no recurrence. The success rate was 78.1% in the 5 mg/ml group, 76.2% in the 10 mg/ml group, and 78.4% in the 40 mg/ml group. These results were not statistically significant (p = 0.999, Fisher's exact test). Yellow deposits developed in 4 out of 37 lesions in the 40mg/ml group, and in 1 out of 42 lesions in the 10 mg/ml group. Skin depigmentation was observed in 1 case in the 5 mg/ml group.
References
1. Dua HS, Nilawar DV. Nonsurgical therapy of chalazion. Am J Ophthalmol. 1982; 94:424–5.
2. Pizzarello LD, Jakobiec FA, Hofeldt AJ, et al. Intralesional corticosteroid therapy of chalazia. Am J Ophthalmol. 1978; 85:818–21.

3. Ben Simon GJ, Huang L, Nakra T, et al. Intralesional triamcinolone acetonide injection for primary and recurrent chalazia: is it really effective? Ophthalmology. 2005; 112:913–7.

4. Mohan K, Dhir SP, Munjal VP, Jain IS. The use of intralesional steroids in the treatment of chalazion. Ann Ophthalmol. 1986; 18:158–60.
5. Ben Simon GJ, Rosen N, Rosner M, Spierer A. Intralesional triamcinolone acetonide injection versus incision and curettage for primary chalazia: a prospective, randomized study. Am J Ophthalmol. 2011; 151:714–8.

6. Park JH, Son JH. Effectiveness for intralesional triamcinolne acetonide injections for chalazia in pediatric patients. J Korean Ophthalmol Soc. 2009; 50:1295–300.

7. Watson AP, Austin DJ. Treatment of chalazions with injection of a steroid suspension. Br J Ophthalmol. 1984; 68:833–5.

8. Kim YW, Lee JO, Lee HB. Intralegional triamcinolone acetonide therapy of chalazia. J Korean Ophthalmol Soc. 1980; 21:377–9.
9. Park YG, Park YT, Hong KS. Intralesional triamcinolone acetonide therapy of chalazia. J Korean Ophthalmol Soc. 1981; 22:499–502.
11. Thomas EL, Laborde RP. Retinal and choroidal vascular occlusion following intralesional corticosteroid injection of a chalazion. Ophthalmology. 1986; 93:405–7.

12. Hosal BM, Zilelioğlu G. Ocular complication of intralesional corticosteroid injection of a chalazion. Eur J Ophthalmol. 2003; 13:798–9.
13. Smythe D, Hurwitz JJ, Tayfour F. The management of chalazion: a survey of Ontario ophthalmologists. Can J Ophthalmol. 1990; 25:252–5.
14. Khurana AK, Ahluwalia BK, Rajan C. Chalazion therapy. Intralesional steroids versus incision and curettage. Acta Ophthalmol. 1988; 66:352–4.

15. Prasad S, Gupta AK. Subconjunctival total excision in the treatment of chronic chalazia. Indian J Ophthalmol. 1992; 40:103–5.
Table 1.
Demographics of patients diagnosed with chalazion
Table 2.
Demographics according to the concentrations of triamcinolone acetonide
| 5 mg/ml | 10 mg/ml | 40 mg/ml | p-value | |
|---|---|---|---|---|
| Number of lesions | 41 | 42 | 37 | |
| Mean age (years) | 25.4 ± 17.9 | 20.6 ± 20.4 | 20.0 ± 15.6 | 0.162% |
| Sex (n) | ||||
| Male | 18 | 14 | 21 | 0.124† |
| Female | 23 | 28 | 16 | |
Table 3.
Treatment outcome according to the concentrations of triamcinolone acetonide (TA)
| Concentration of TA | Success rates, lesions (%) | p-value* |
|---|---|---|
| 5 mg/ml | 32/41 (78.1) | |
| 10 mg/ml | 32/42 (76.2) | 0.999 |
| 40 mg/ml | 29/37 (78.4) | |
| Total | 93/120 (77.5) |
Table 4.
The number of lesions that developed yellow deposits according to the concentrations of triamcinolone acetonide (TA)
| Concentration of TA | The number of yellow deposits (%) | p-value* |
|---|---|---|
| 5 mg/ml | 0/41 (0) | |
| 10 mg/ml | 1/42 (2.1) | 0.039 |
| 40 mg/ml | 4/37 (10.8) | |
| Total | 5/120 (4.2) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download