Abstract
Purpose
To evaluate the efficacy of cyclosporine 0.05% in reduced tear production sign and dry eye symptoms after cata-ract surgery.
Methods
This hospital-based prospective randomized trial included 43 patients of 83 eyes who underwent phacoemulsification. Tear break-up time, Schirmer’s test, corneal and conjunctival stain, and ocular surface disease index were performed for all pa-tients at preoperative 1 day, and 1 day, 1 week, 1 month, and 2 months postoperatively. Group 1 received carboxymethylcellulose 0.5%, group 2 received twice-daily cyclosporine 0.05%, and group 3 did not receive any additional eye drops.
References
1. Begley CG, Caffery B, Nichols K, et al. Results of a dry eye ques-tionnarire from optometric practices. Adv Exp Med Biol. 2002; 506(Pt B):1009–16.
2. Holly FJ. Surface chemical evaluation of artificial tears and their ingredients, II. Interaction with a superficial lipid layer. Eye Contact Lens. 1978; 4:52–65.
3. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007; 26(9 Suppl 1):16–20.

4. Oh T, Jung Y, Chang D, et al. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. 2012; 56:113–8.

5. Hoffman RS, Fine IH, Packer M. New phacoemulsification technology. Curr Opin Opthalmol. 2005; 16:38–43.

6. Khanal S, Tomlinson A, Esakowitz L, et al. Changes in corneal sen-sitivity and tear physiology after phacoemulsification. Ophthalmic Physiol Opt. 2008; 28:127–34.

7. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine oph-thalmic emulsion in moderate to severe dry eye disease. the CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–9.
8. Donnenfeld ED, Solomon R, Roberts CW, et al. Cyclosporine 0.05% to improve visual outcomes after multifocal intraocular lens implantation. J Cataract Refract Surg. 2010; 36:1095–100.

9. Schhiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000; 118:615–21.

10. Preschel N, Hardten DR. Management of coincident corneal dis-ease and cataract. Curr Opin Ophthalmol. 1999; 10:59–65.

11. Mathers WD. Why the eye becomes dry: a cornea and lacrimal gland feedback model. CLAO J. 2000; 26:159–65.
12. Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion of soft tissues. J Controll Release. 1985; 2:257–75.
13. Hunt G, Kearney P, Kellaway IW. Mucoadhesive polymers in drug delivery systems fundamentals and techniques. Weinheim:: Germany VCH;1987. p. 180–99.
14. Lee JH, Ahn HS, Kim EK, Kim TI. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea. 2011; 30:175–9.

15. Grene RB, Lankston P, Mordaunt J, et al. Unpreserved carbox-ymethylcellulose artificial tears evaluated in patients with kerato-conjunctivitis sicca. Cornea. 1992; 11:294–301.

16. Byun YS, Rho CR, Cho K, et al. Cyclosporine 0.05% ophthalmic emulsion for dry eye in Korea: a prospective, multicenter, open-la-bel, surveillance study. Korean J Ophthalmol. 2011; 25:369–74.

17. Byun YJ, Kim TI, Seo KY. The short-term effect of topical cyclo-sporine 0.05% in various ocular surface disorder. J Korean Ophthalmol Soc. 2008; 49:401–8.
18. Preschel N, Hardten DR. Management of coincident corneal dis-ease and cataract. Curr Opin Ophthalmol. 1999; 10:59–65.

19. Pflugfelder SC, Solomon A, Stern ME. The diagnosis and manage-ment of dry eye: a twenty-ive-year review. Cornea. 2000; 19:644–9.
20. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–9.
Figure 1.
Tear Break up time (TBUT) at pre-op and post-op 1 day, 1 week, 1 month, and 2 months. The increase in TBUT was statistically significant between1 month and 2 months (Group 1 = Carboxymethylcellulose 0.5%, Group 2 = Cyclosporine 0.05%, Group 3 = No additional eye drops).
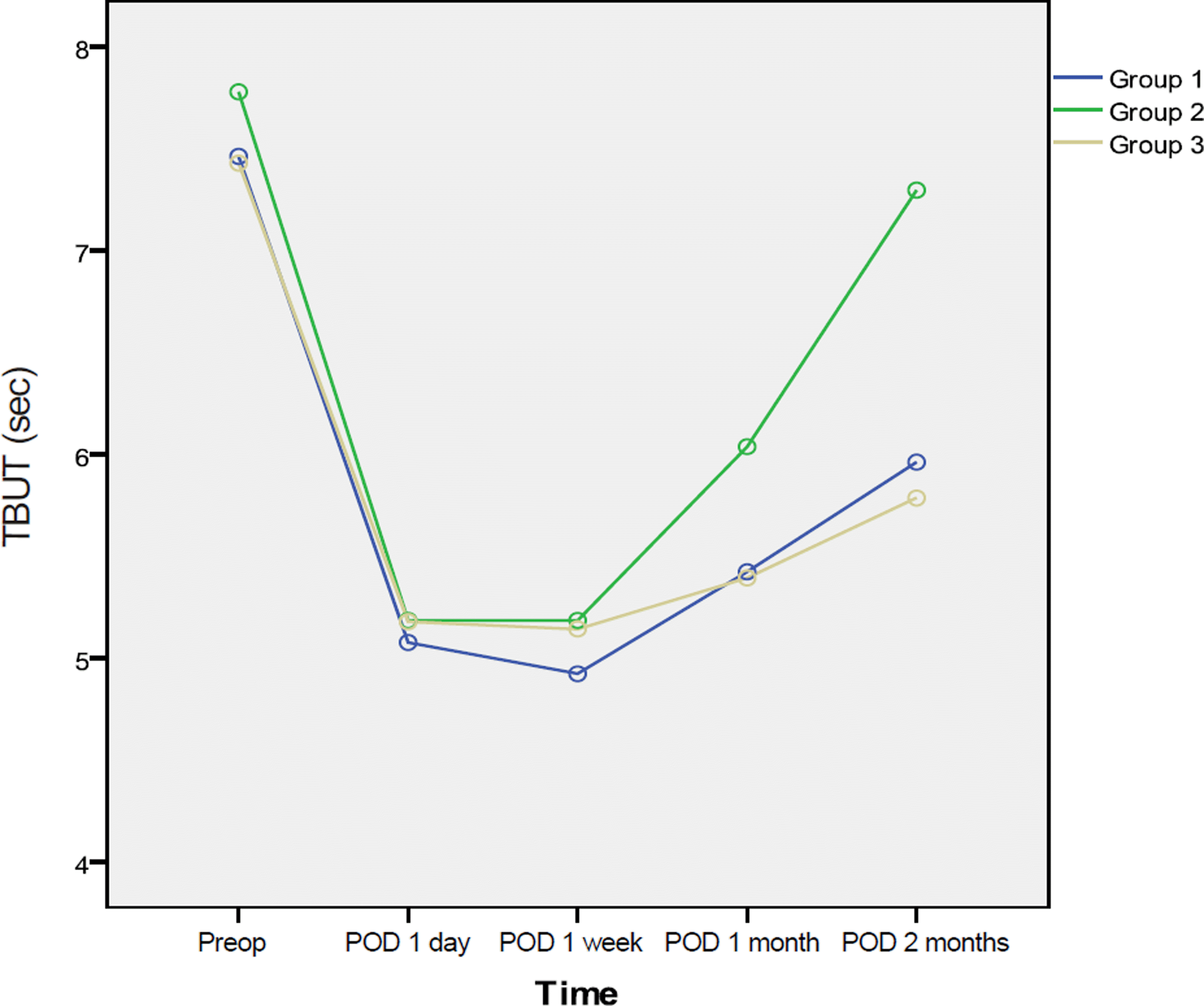
Figure 2.
Schirmer test I at pre-op and post-op 1 day, 1 week, 1 month, and 2 months. Schirmer test I of group 2 increased statistically significant between post-op 1 month and 2 months (Group 1 = Carboxymethylcellulose 0.5%, Group 2 = Cyclosporine 0.05%, Group 3 = No additional eye drops).
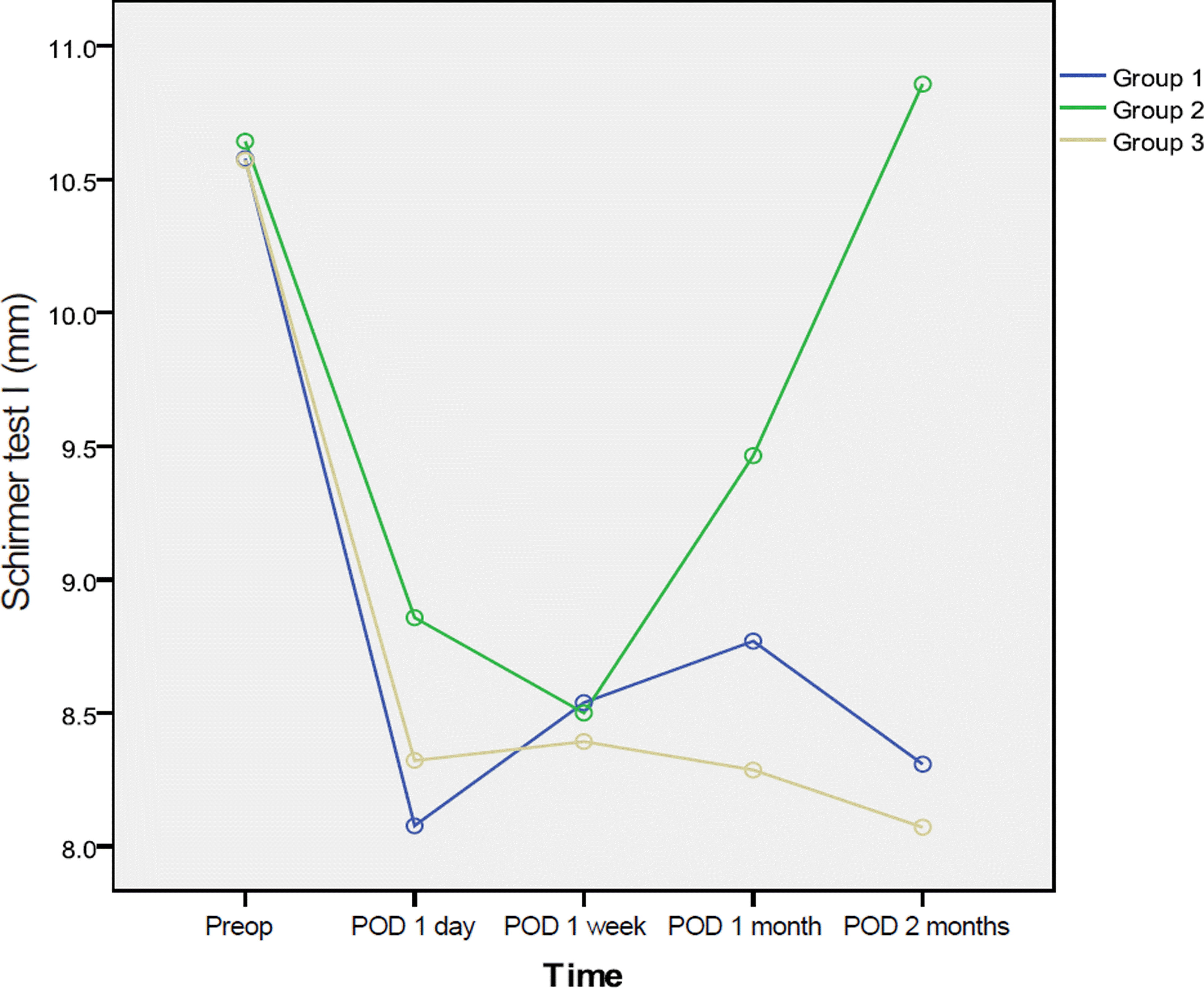
Figure 3.
Corneal and conjunctival staining at pre-op and post-op 1 month, 2 months. Group 2 had lowered corneal and conjunctival staining grades 2 months after cataract surgery (Group 1 = Carboxymethylcellulose 0.5%, Group 2 = Cyclos- porine 0.05%, Group 3 = No additional eye drops).
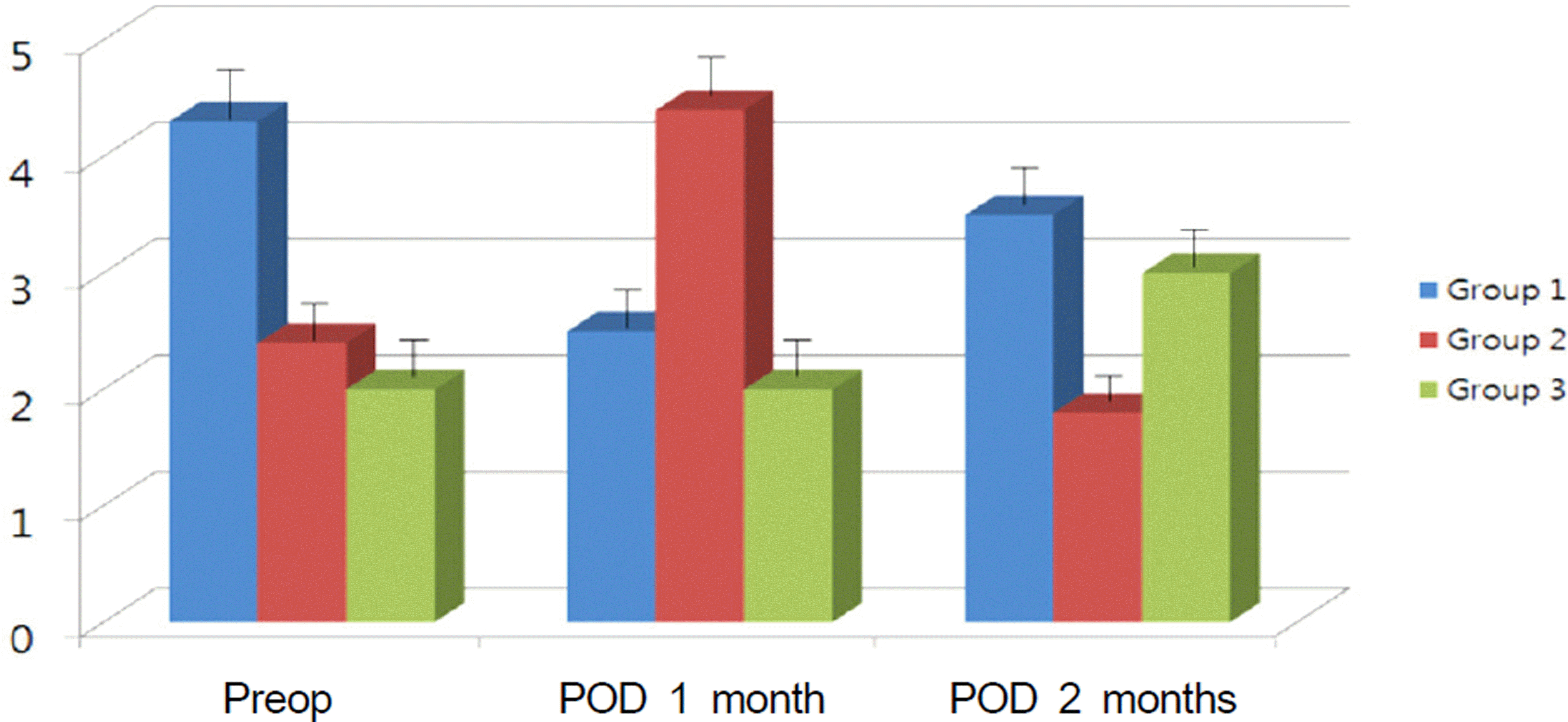
Figure 4.
Mean ocular surface disease index (OSDI) score at pre-op and after surgery. The OSDI score decreased more in group1 and 2 than group 3 from 1 week after surgery to 2 month (Group 1 = Carboxymethylcellulose 0.5%, Group 2 = Cyclosporine 0.05%, Group 3 = No additional eye drops).
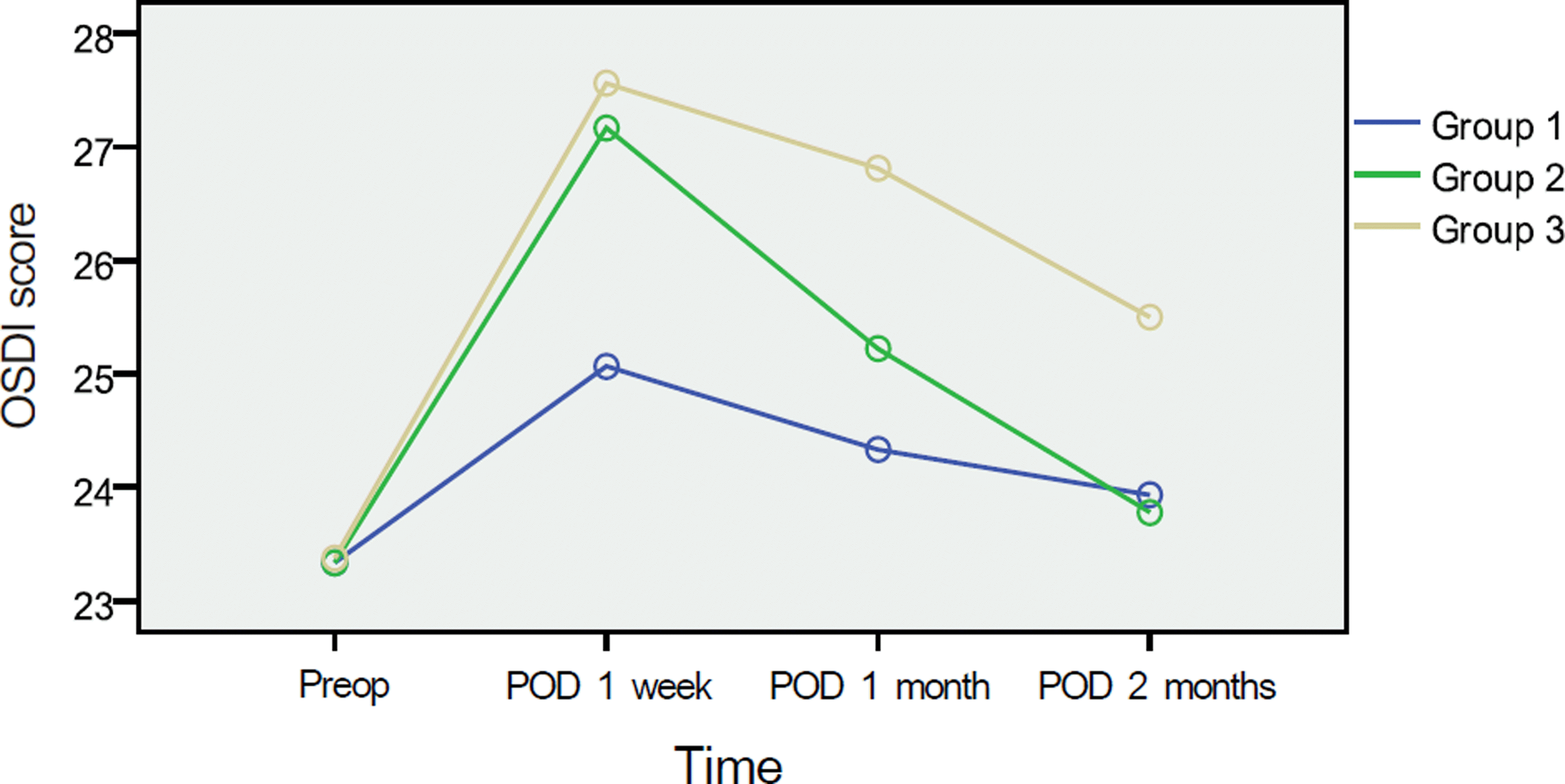
Table 1.
Baseline data by treatment
| Parameter | Group 1* | Treatment Group 2† | Group 3‡ | p-value |
|---|---|---|---|---|
| Tear film break up time | 7.46 ± 1.5 | 7.75 ± 1.4 | 7.43 ± 1.7 | 0.704 |
| Schirmer test I | 10.58 ± 2.5 | 10.59 ± 2.5 | 10.57 ± 2.4 | 1.000 |
| Corneal & conjunctival stain | 1.77 ± 0.15 | 1.83 ± 0.18 | 1.64 ± 0.17 | 0.760 |
| Ocular surface disease index | 23.33 ± 4.3 | 23.38 ± 4.1 | 23.38 ± 4.4 | 0.085 |
Table 2.
Tear film break up time
| Parameter | Treatment | p-value | ||
|---|---|---|---|---|
| Group 1* | Group 2† | Group 3‡ | ||
| POD 1 day | 5.08 ± 2.01 | 5.19 ± 1.97 | 5.18 ± 2.10 | 0.881 |
| POD 1 week | 5.10 ± 2.03 | 5.22 ± 1.85 | 5.23 ± 2.13 | 0.781 |
| POD 1 month | 5.42 ± 2.01 | 6.04 ± 2.03 | 5.39 ± 2.21 | 0.006 |
| POD 2 months | 5.79 ± 1.98 | 7.29 ± 2.01 | 5.96 ± 1.98 | 0.003 |
Table 3.
Schirmer test I
| Parameter | Treatment | p-value | ||
|---|---|---|---|---|
| Group 1* | Group 2† | Group 3‡ | ||
| POD 1 day | 8.77 ± 2.4 | 9.46 ± 2.2 | 8.29 ± 2.1 | 0.133 |
| POD 1 week | 8.56 ± 2.1 | 8.87 ± 2.3 | 8.33 ± 2.2 | 0.147 |
| POD 1 month | 8.52 ± 2.3 | 9.52 ± 2.3 | 8.32 ± 2.2 | 0.152 |
| POD 2 months | 8.30 ± 2.5 | 10.83 ± 2.2 | 8.07 ± 2.1 | 0.003 |
Table 4.
Corneal & Conjunctival staining
| Parameter | Treatment | p-value | ||
|---|---|---|---|---|
| Group 1* | Group 2† | Group 3‡ | ||
| POD 1 day | 1.08 ± 0.22 | 1.17 ± 0.21 | 1.17 ± 0.17 | 0.142 |
| POD 1 week | 1.12 ± 0.14 | 1.24 ± 0.18 | 1.17 ± 0.15 | 0.130 |
| POD 1 month | 1.32 ± 0.17 | 1.25 ± 0.15 | 1.22 ± 0.19 | 0.152 |
| POD 2 months | 1.42 ± 0.15 | 0.88 ± 0.14 | 1.33 ± 0.17 | 0.004 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download