Abstract
Purpose
To evaluate the efficacy and safety of laser photocoagulation and intravitreal injection of bevacizumab in zone I ROP (retinopathy of prematurity) accompanied with plus signs.
Methods
We performed a retrospective analysis of the records of 12 eyes of 7 premature infants with zone I ROP accompanied with plus signs, treated with diode laser photocoagulation and intravitreal injection of bevacizumab that were followed-up for at least 12 months.
Results
Mean gestational age was 30 + 2 weeks, mean birth weight was 1437 ± 478 g, mean follow-up period was 14.7 ± 2.0 months and mean age of diagnosis was 36 + 3 weeks. Plus signs were disappeared after an average of 10.1 ± 2.4 postoperative days in all 12 eyes. The outcome was favorable in 11 (91.7%) of 12 treated eyes after a minimum of 12 months of follow-up. No local or systemic complications were observed.
Figures and Tables
Figure 1
Case 2. Fundus findings at preoperative (A, B) and 12 days after diode laser photocoagulation and intravitreal bevacizumab injection (C, D). (A, C) Right eye, (B, D) left eye. (A, B) Zone I retinopathy of prematurity with severe plus disease and flat neovascularization. (C, D) Note retinopathy of prematurity regression with resolution of plus disease. More continued peripheral vascularization over the laser scar in temporal retina.
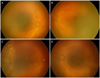
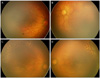
Figure 2
Case 5. Fundus findings at preoperative (A, B) and 10 days after diode laser photocoagulation and intravitreal bevacizumab injection (C, D). (A, C) Right eye, (B, D) left eye. (A, B) Zone I retinopathy of prematurity with very severe plus disease and severe flat neovascularization. (C, D) Note resolution of plus disease and flat neovascularization. More continued peripheral vascularization in temporal retina.
References
1. Terry TL. Fibroblastic Overgrowth of Persistent Tunica Vasculosa Lentis in Infants Born Prematurely: II. Report of Cases-Clinical Aspects. Trans Am Ophthalmol Soc. 1942. 40:262–284.
2. Kinsey VE. Retrolental fibroplasia; cooperative study of retrolental fibroplasia and the use of oxygen. AMA Arch Ophthalmol. 1956. 56:481–543.
3. Lanman JT, Guy LP, Dancis J. Retrolental fibroplasia and oxygen therapy. J Am Med Assoc. 1954. 155:223–226.
4. Kychenthal A, Dorta P, Katz X. Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina. 2006. 26:7 Suppl. S11–S15.
5. Cryotherapy for retinopathy of prematurity cooperative group. Multicenter trial of cryotherapy for retinopathy of prematurity. Three-month outcome. Arch Ophthalmol. 1990. 108:195–204.
6. Early Treatment For Retinopathy Of Rrematurity Cooperative Group. Revised indication for the treatment of retinopathy of prematurity: results of early treatment for ROP randomized trial. Arch Ophthalmol. 2003. 121:1684–1696.
7. Flynn JT, Chan-Ling T. Retinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol. 2006. 142:46–59.
8. Katz X, Kychenthal A, Dorta P. Zone I retinopathy of prematurity. J AAPOS. 2000. 4:373–376.
9. O'Keefe M, Lanigan B, Long VW. Outcome of zone I retinopathy of prematurity. Acta Ophthalmol Scand. 2003. 81:614–616.
10. Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology. 2009. 116:1599–1603.
11. Nonobe NI, Kachi S, Kondo M, et al. Concentration of vascular endothelial growth factor in aqueous humor of eyes with advanced retinopathy of prematurity before and after intravitreal injection of bevacizumab. Retina. 2009. 29:579–585.
12. Gordon MS, Cunnigham D. Managing patients treated with bavacizumab combination therapy. Oncology. 2005. 69:Suppl 3. 25–33.
13. Skilling JR, Johnson DH, Miller K, et al. Arterial thromboembolic events (ATEs) is a pooled analysis of 5 randomized, controlled trials (RCTs) of bevacizumab (BV) with chemotherapy. J Clin Oncol. 2005. 23:S196.
14. Seo JW, Park IW. Intravitreal bevacizumab for treatment of diabetic macular edema. Korean J Ophthalmol. 2009. 23:17–22.
15. Velez-Montoya R, Fromow-Guerra J, Burgos O, et al. The effect of unilateral intravitreal bevacizumab (avastin), in the treatment of diffuse bilateral diabetic macular edema: a pilot study. Retina. 2009. 29:20–26.
16. Chung EJ, Hong YT, Lee SC, et al. Prognostic factors for visual outcome after intravitreal bevacizumab for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2008. 246:1241–1247.
17. Kusaka S, Shima C, Wada K, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol. 2008. 92:1450–1455.
18. Mintz-Hittner HA, Kuffel RR. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008. 28:831–838.
19. Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995. 15:4738–4747.
20. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005. 123:991–999.
21. Hardy RJ, Good WV, Dobson V, et al. Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials. 2004. 25:311–325.
22. Coats DK, Miller AM, Brady McCreery KM, et al. Involution of threshold retinopathy of prematurity after diode laser photocoagulation. Ophthalmology. 2004. 111:1894–1898.
23. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity, preliminary results. Arch Ophthalmol. 1988. 106:471–479.
24. Robinson R, O'Keefe M. Cryotherapy for retinopathy of prematurity--a prospective study. Br J Ophthalmol. 1992. 76:289–291.
25. Gunn TR, Easdown J, Outerbridge EW, Aranda JV. Risk factors in retrolental fibroplasias. Pediatrics. 1980. 65:1096–1100.
26. Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003. 110:1690–1696.
27. Funatsu H, Yamashita H, Sakata K, et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005. 112:806–816.
28. Barakat MR, Kaiser PK. VEGF inhibitors for the treatment of neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2009. 18:637–646.
29. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997. 18:4–25.
30. Shah PK, Narendran V, Tawansy KA, et al. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007. 55:75–76.
31. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007. 245:1727–1730.
32. Lee JY, Chae JB, Yang SJ, et al. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2010. 248:1257–1262.
33. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005. 112:1035–1047.
34. Wu WC, Lai CC, Chen KJ, et al. Long-term tolerability and serum concentration of bevacizumab (avastin) when injected in newborn rabbit eyes. Invest Ophthalmol Vis Sci. 2010. 51:3701–3708.
35. Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2008. 26:399–405.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download