Abstract
Purpose
The present study compares, using a new generation high‐ resolution in vivo confocal microscope, the corneas of patients who underwent photorefractive keratectomy (PRK) 10 years previously with those of healthy persons.
Case summary
A confocal microscope (Confoscan 4.0, Fortune Technology, Italy) was used to get the data from healthy volunteers and patients. Corneal cross‐ sectional images of the epithelium, Bowman’s layer, stromal layer (anterior, middle and posterior keratocyte), Descemet’s membrane, and endothelium were compared. In PRK corneas, the superficial epithelium was nearly intact and the subbasal nerve plexus was visible, but some hyperreflective areas were also found in the nerve plexus. Because of the absence of the Bowman’s layer, some ECM and keratocytes were visualized in their optical section. Although anterior keratocytes showed uneven distribution with less cellularity, middle and posterior keratocytes looked unaffected. Likewise, there were no differences in the endothelium between the two groups.
References
1. Trokel SL, Srinnivasan R, Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983; 96:710–5.

2. Epstein D, Fagerholm P, Hamberg-Nyström H, Tengroth B. Twenty-four month follow-up of excimer laser photorefractive keratectomy for myopia: refractive and visual acuity results. Ophthalmology. 1994; 101:1558–63.
3. Dutt S, Steinert RF, Raizman MB, Puliafito CA. One-year results of excimer laser photorefractive keratectomy for low to moderate myopia. Arch Ophthalmol. 1994; 112:1427–36.

4. Kim JH, Kim MS, Hahn TW, et al. Five year results of photo-refractive keratectomy for myopia. J Cataract Refract Surg. 1997; 23:731–5.

5. Stephenson CG, Gartry DS, O'Brart DP, et al. Photorefractive keratectomy: a 6-year follow-up study. Ophthalmology. 1998; 105:273–81.
6. Moilanen JA, Vesaluoma MH, Müller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003; 44:1064–9.

7. Erie JC, McLaren JW, Hodge DO, Bourne WM. Long-term corneal keratoctye deficits after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2005; 103:56–68.
8. Rosenberg ME, Tervo TM, Petroll WM, Vesaluoma MH. In vivo confocal microscopy of patient with corneal recurrent erosion syndrome or epithelial basement membrane dystrophy. Ophthalmology. 2000; 107:565–73.
9. Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in typeⅠ diabetes mellitus. Invest Ophthalmol Vis Sci. 2000; 41:2915–21.
10. Linna T, Tervo T. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997; 16:640–49.

11. Frueh BE, Cadez R, Böhnke M. In vivo confocal microscopy after photorefractive keratectomy in humans: a prospective, long-term study. Arch Ophthalmol. 1998; 116:1425–31.
12. Erie JC. Corneal wound healing after photorefractive keratectomy: a 3-year confocal microscopy study. Trans Am Ophthalmol Soc. 2003; 101:287–328.
13. Ozdamar A, Aras C, Karakas N, et al. Changes in tear flow and tear film stability after photorefractive keratectomy. Cornea. 1999; 18:437–9.

14. Seiler T, McDonnell P. Excimer laser photorefractive keratectomy. Surv Ophthalmol. 1995; 40:89–118.

15. Li DQ, Tseng SC. Three patterns of cytokine expression potentially invlolved in epithelial-fibroblast interactions of human ocular surface. J Cell Phys. 1995; 163:61–79.
16. Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000; 107:1235–45.

17. Lohmann C, Gartry D, Kerr Muir M, et al. “Haze” in photorefractive keratectomy; its origins and consequences. Laser Light Ophthalmol. 1991; 4:15–34.
Figure 1.
Confocal microscopic images of the subbasal nerve plexus layer of the cornea in the control (A) and corresponding post-PRK corneas (B). (A) Normal subbasal nerve plexus. (B) In post-PRK corneas, the anterior keratocyte and some hyperreflective ECM were imaged together with regenerated nerve plexus (arrow).
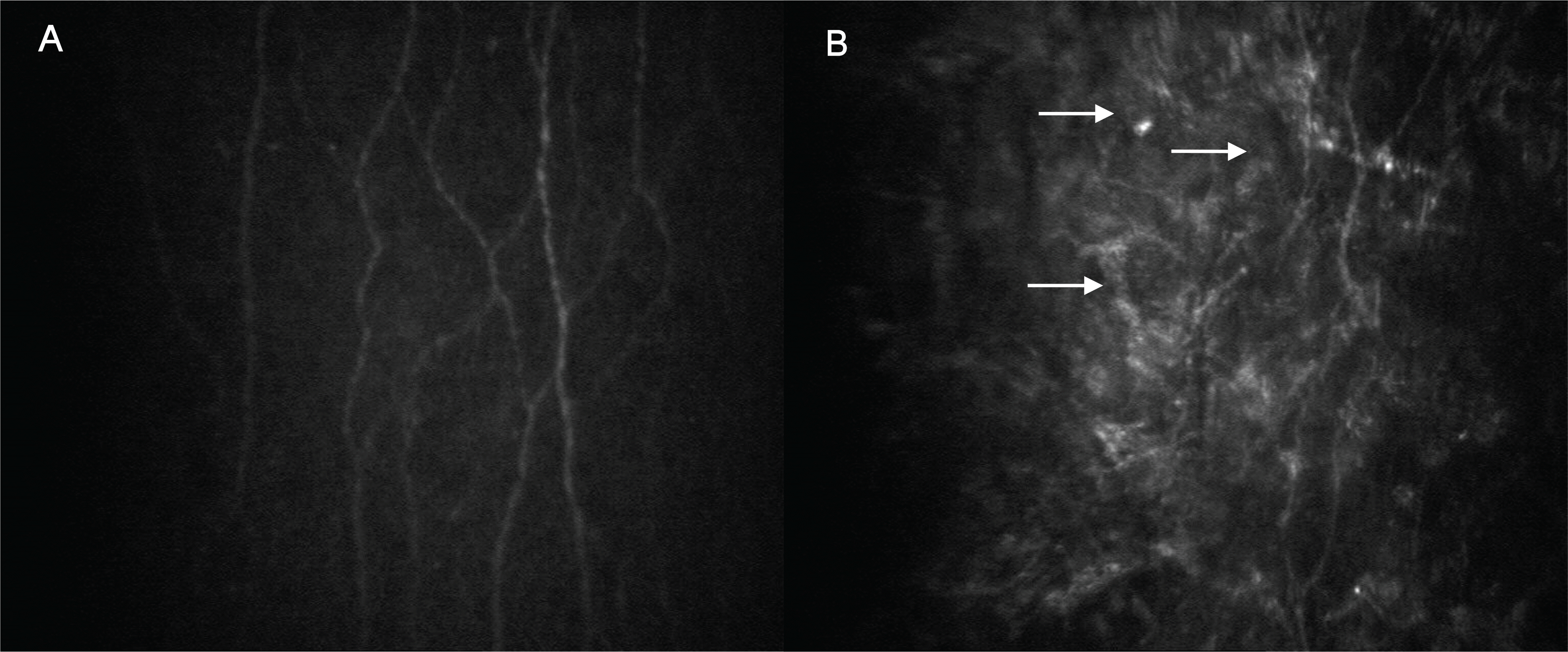
Figure 2.
Confocal microscopic images of the anterior stromal layer of the cornea in the control (A) and corresponding post-PRK corneas (B). (A) Evenly distributed anterior keratocytes. (B) Unevenly distributed anterior keratocytes (arrow) showed decreased cellular density and some hyperreflectivity.
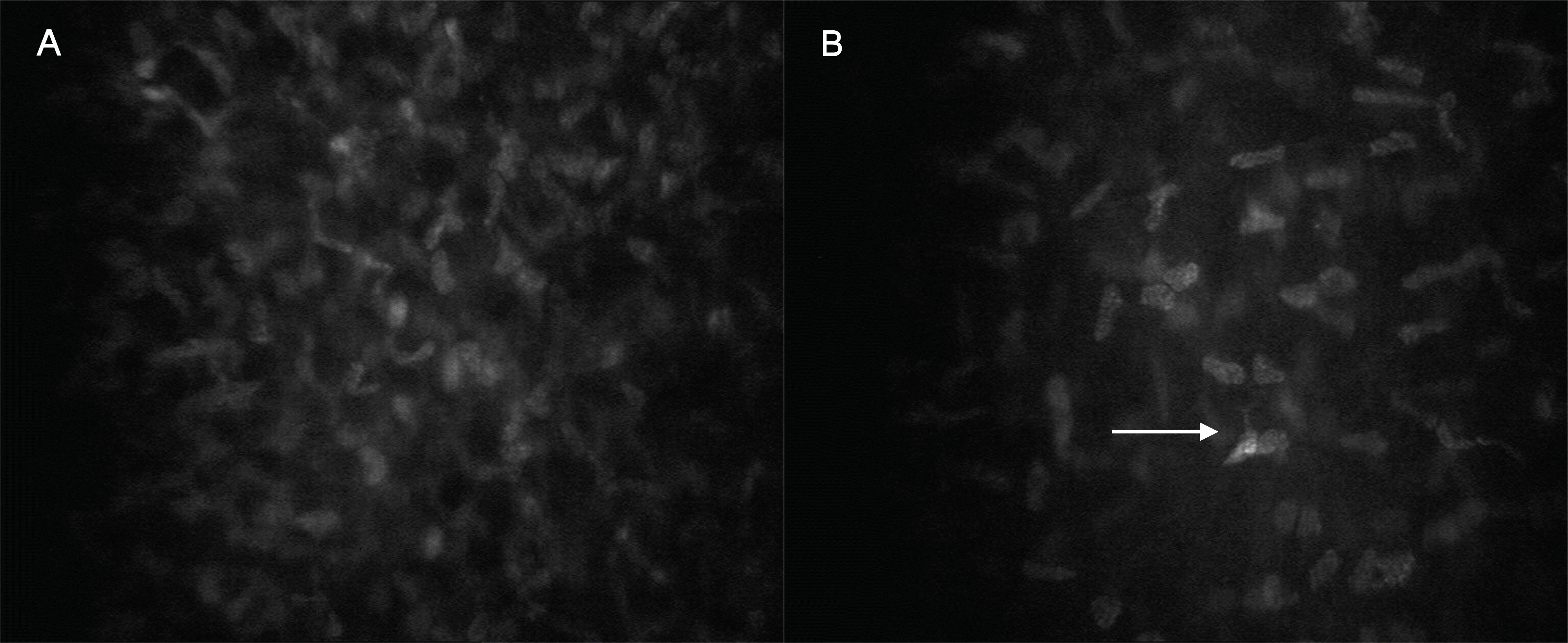
Figure 3.
Confocal microscopy images of the middle and posterior stromal layer of the cornea in the control (A, C) and corresponding post-PRK corneas (B, D). (A) Midstromal keratocytes. (B) Only minor changes were observed in the post-PRK midstroma. (C, D) The posterior cornea appeared unaffected in both groups.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download