Abstract
Purpose
To evaluate the short-term effects of a single intravitreal injection of bevacizumab (Avastin) for the management of new vessels (NV) associated with proliferative diabetic retinopathy (PDR).
Methods
A non-randomized study of 19 PDR patients (20 eyes) who had active NV was analyzed prospectively. Standardized ophthalmic evaluation was performed at baseline and at weeks 1, 4, and 8 after intravitreal injection of 1.25 mg of bevacizumab. The main outcome measures included changes in total area of fluorescein leakage from active NV and best corrected visual acuity (BCVA).
Results
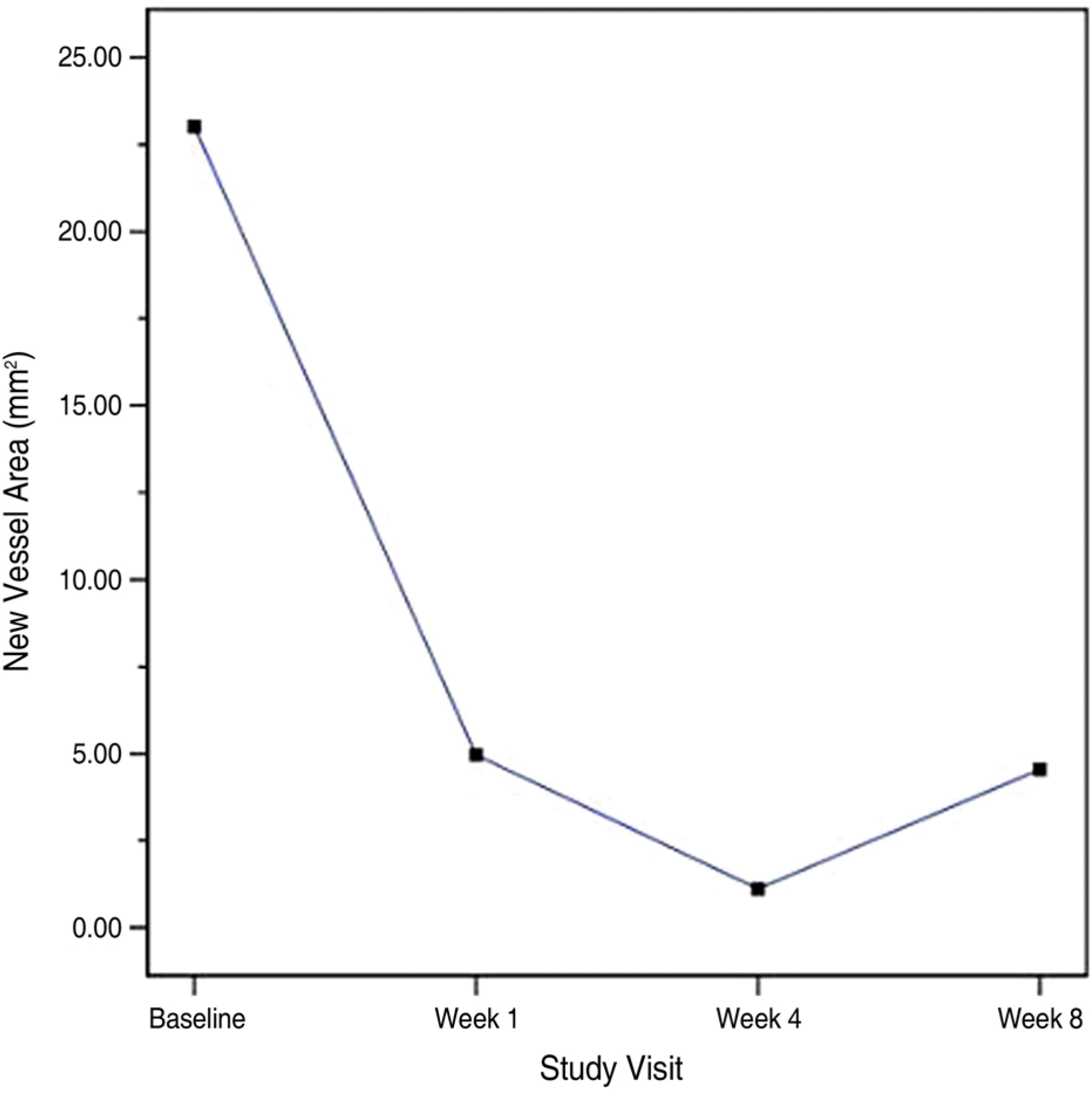
Twenty eyes of 19 patients (12 men [63.2%], 7 women [36.8%]) were included and all patients completed the 8-week study followup period. The mean age of participants was 47.05±12.48 years. At baseline, NV area was 23.02±21.80 mm2. The area of active NV decreased significantly to 4.96±9.18 mm2, 1.11±4.96 mm2 and 4.55±5.11 mm2 (p<0.05) at 1, 4 and 8 weeks after injection, respectively. At week 4, no leakage was observed in 19 eyes. The mean logMAR BCVA improved from 0.59±0.49 at baseline to 0.56±0.47, 0.55±0.73 and 0.51±0.50 at weeks 1, 4, and 8, respectively. No significant adverse events were observed.
References
1. Michaelson IC. The mode of development of the vascular system of the retina, with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc U K. 1948; 68:137–80.
3. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

4. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005; 25:111–8.

5. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

6. Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996; 103:1820–8.

7. Tolentino MJ, Miller JW, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhumanprimate. Arch Ophthalmol. 1996; 114:964–70.
8. Vander JF, Duker JS, Benson WE, et al. Long-term stability and visual outcome after favorable initial response of proliferative diabetic retinopathy to panretinal photocoagulation. Ophthalmology. 1991; 98:1575–9.

9. Flynn HW Jr, Chew EY, Simons BD, et al. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. Ophthalmology. 1992; 99:1351–7.
10. The Diabetic Retinopathy Study Group. Photocoagulation for diabetic treatment of proliferative diabetic retinopathy: Clinical application of DRS Findings. Ophthalmology. 1981; 88:583–600.
11. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991; 98:766–85.
12. Shimura M, Yasuda K, Nakazawa T, et al. Quantifying alterations of macular thickness before and after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Ophthalmology. 2003; 110:2386–94.

13. Macugen Diabetic Retinopathy Study Group. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005; 112:1747–57.
14. Macugen Diabetic Retinopathy Study Group. Changes in retinal neovascularization following pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006; 113:23–8.
15. Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995; 92:10457–61.

16. Martin SS, Efdal Y, Ana S, et al. Comparative antiproliferative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1837–42.

17. Bakri SJ, Snyder MR, Pulido JS, et al. Six-month stability of Bevacizumab (Avastin) binding to vascular endothelial growth factor after withdrawal into a syringe and refrigeration or freezing. Retina. 2006; 26:519–22.

18. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal Bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006; 113:1695.

19. Jorge R, Costa RA, Calucci D, et al. Intravitreal Bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina. 2006; 26:1006–13.

20. Arevalo JF, Maia M, Flynn HW Jr, et al. Tractional retinal detachment following intravitreal Bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008; 92:213–6.

21. Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002; 133:373–85.

22. Adamis AP, Altaweel M, Bressler NM, et al. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006; 113:23–8.

23. Mordenti J, Thomsen K, Licko V, et al. Intraocular pharmacokinetics and safety of a humanized monoclonal antibody in rabbits after intravitreal administration of a solution or a PLGA microsphere formulation. Toxicol Sci. 1999; 52:101–6.

24. Jaissle GB, Ziemssen F, Petermeier K, et al. Bevacizumab for treatment of macular edema secondary to retinal vein occlusion. Ophthalmology. 2006; 10:216–21.
25. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal Bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.
Figure 1.
Setting dimensions of the optic disc. A and B, Measurement of optic disc transverse length (red line). C and D, Measurement of optic disc vertical length (red line).

Figure 2.
Setting extension of new vessels. Red free photographs from diabetic patient with active new vessels taken at baseline (A), and at 8 week (B) after intravitreal bevacizumab injection.
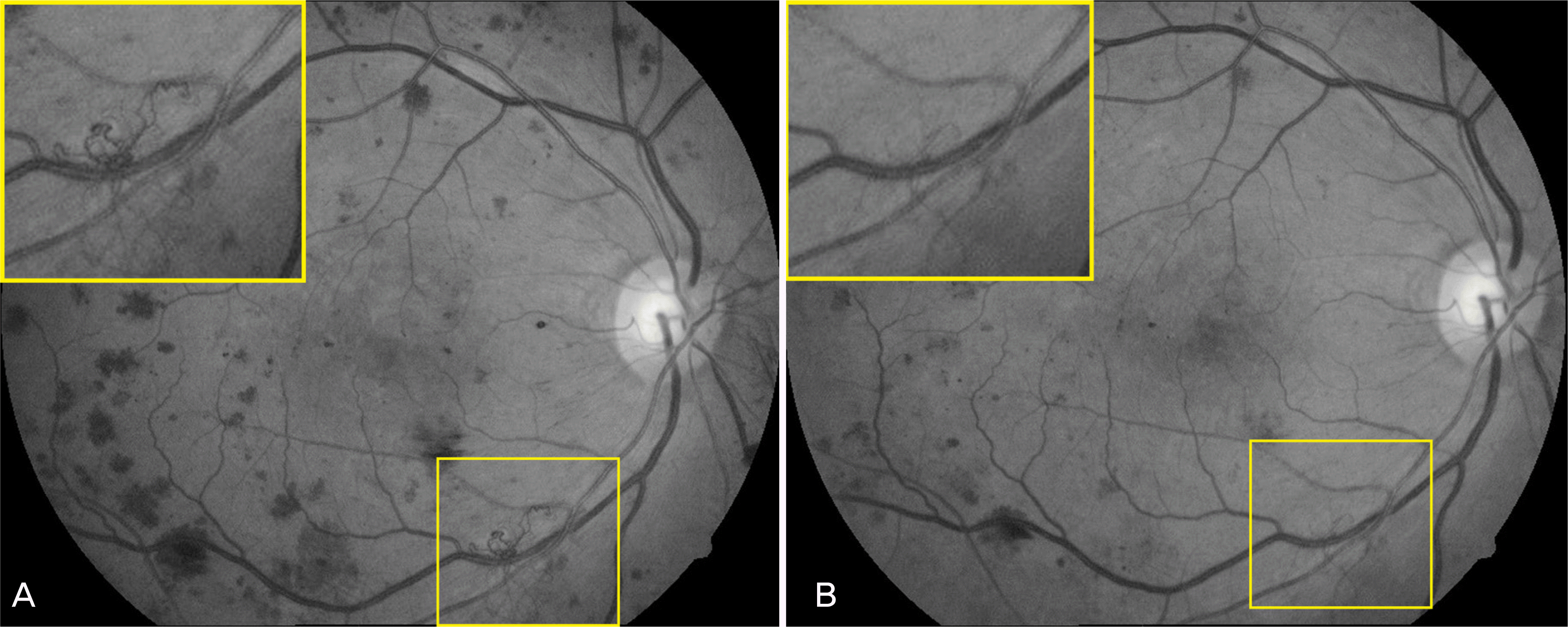
Figure 3.
Setting extension (yellow line) and pixels (red line) of optic disc (A) and new vessel (B, C) by using Adobe® Photoshop® 8.0.
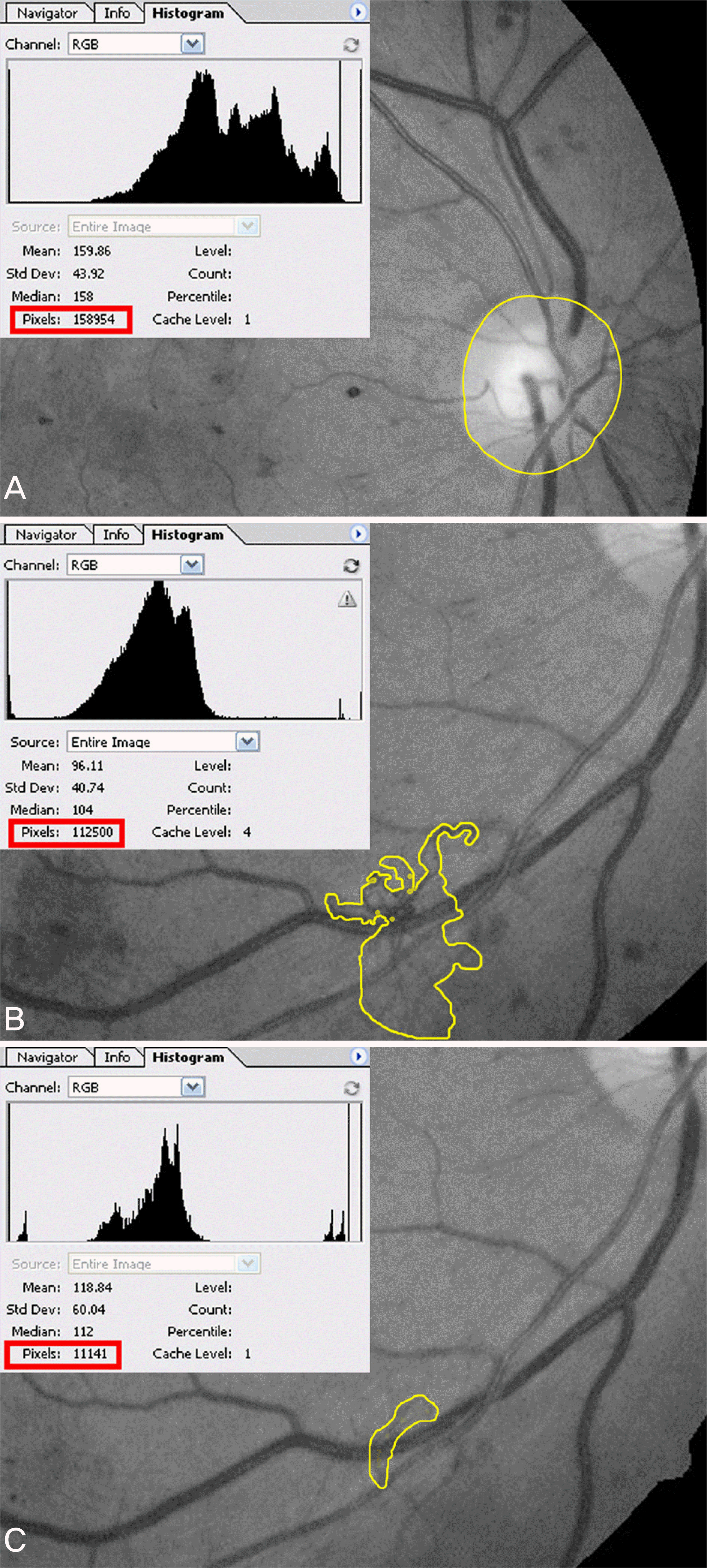
Figure 4.
Best corrected visual acuity (logarithm of the minimum angle of resolution values) after intravitreal bevacizumab injection.
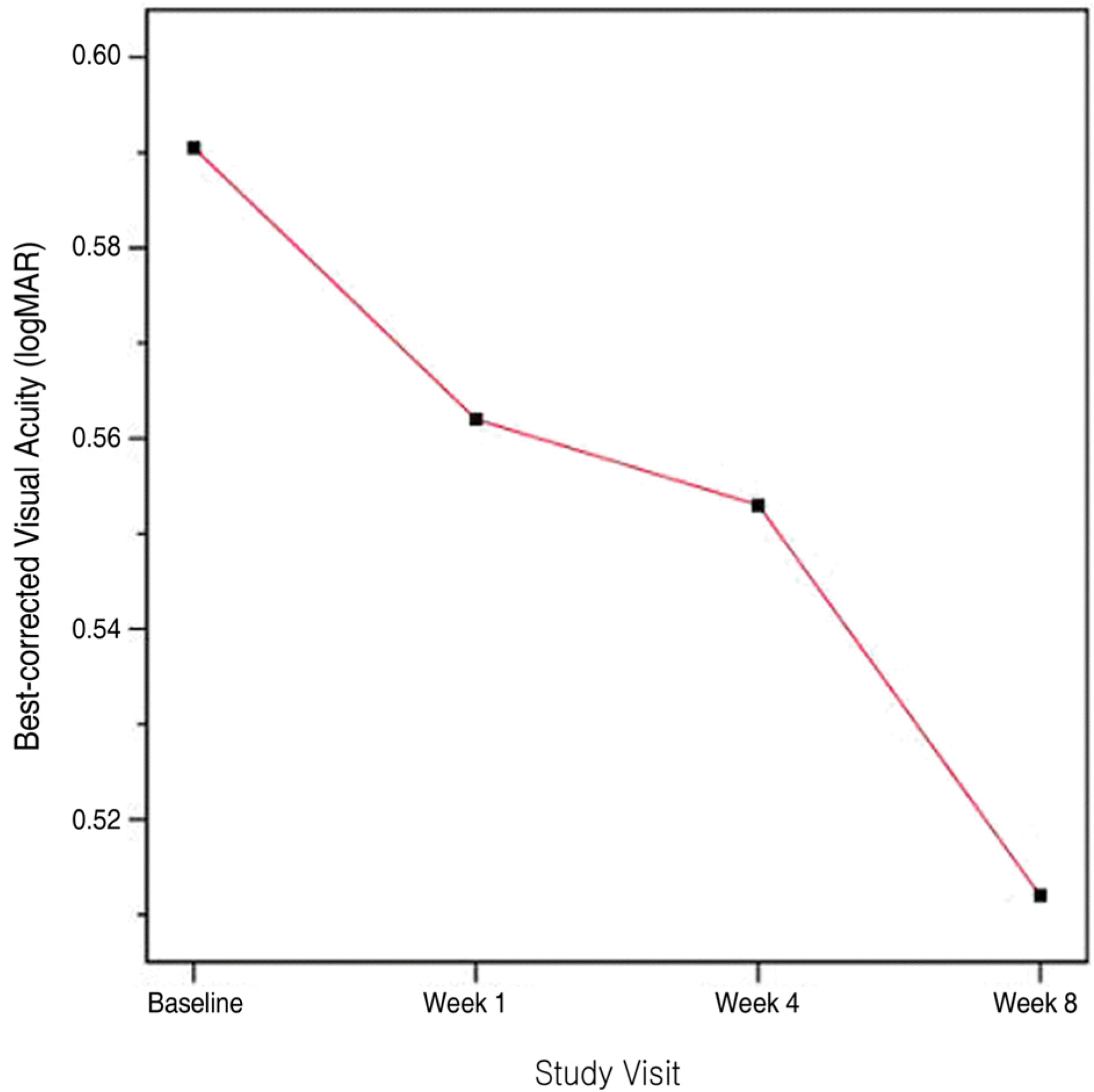
Figure 6.
Color fundus photographs from diabetic patient (case 1) with active new vessels at the disc (NVD) taken at baseline (A), and at 1 week (B), 4 week (C), 8 week (D) after intravitreal bevacizumab injection. Remarkable regression of NVD observed at baseline is seen at week 1, 4, 8 week after one single intravitreal injection of 1.25 mg of bevacizumab.

Figure 7.
Color fundus photographs, red free, early- and late-phase fluorescein angiographs from a diabetic patient (case 2) with active new vessels and fibrovascular proliferation. Leaking new vessels and fibrovascular proliferation was seen at baseline (A). Some staining but no fluorescein leakage from new vessels noted at baseline could be seen at 4 week after an intravitreal injection of 1.25 mg of bevacizumab (B). 8 week after injection minimal fluorescein leakage is observed from new vessels noted at baseline (C).

Figure 8.
Color fundus photographs, red free, early- and late-phase fluorescein angiographs from a diabetic patient (case 4) with active new vessels. Actively leaking new vessels was seen at baseline (A). Some staining but no fluorescein leakage from new vessels noted at baseline could be seen at 4 week after an intravitreal injection of 1.25 mg of bevacizumab (B).
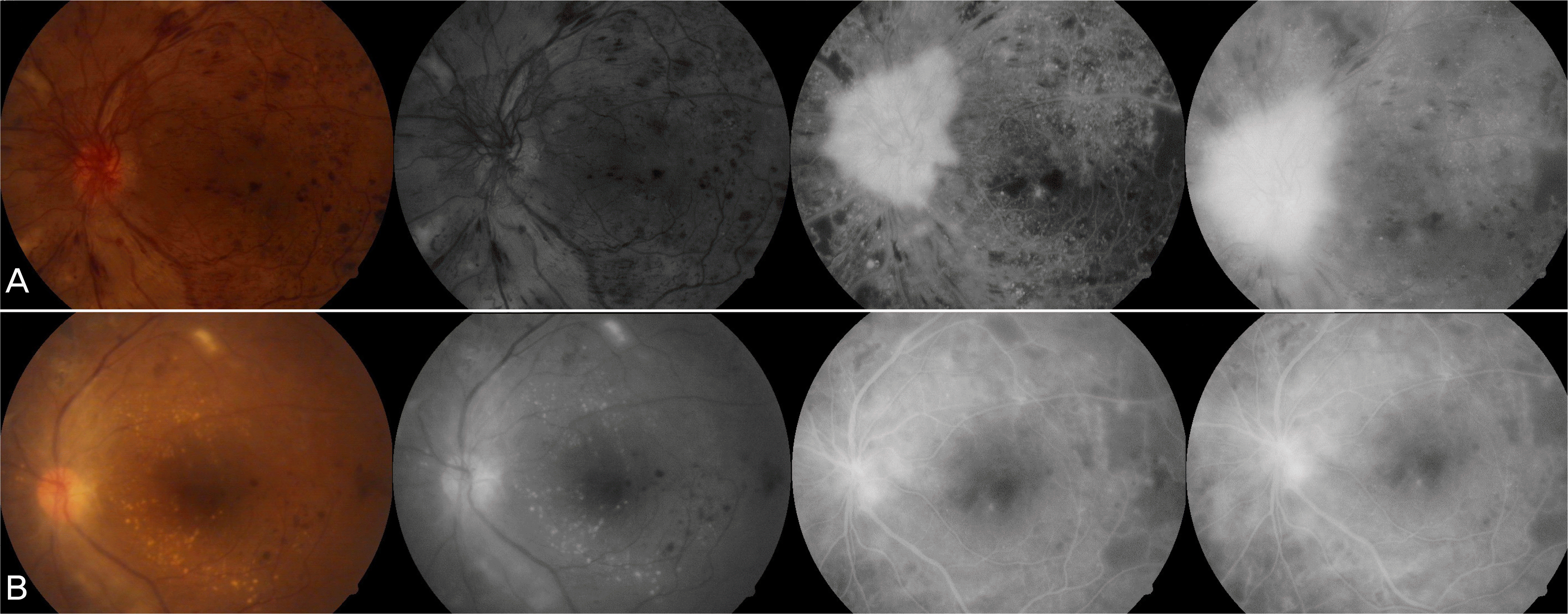
Figure 9.
Color fundus photographs, red free, early-, mid- and late-phase fluorescein angiographs from a diabetic patient (case 8) with active new vessels. Active areas of neovascularization elsewhere was seen at baseline (A). Complete resolution of the fluorescein leakage from new vessels noted at baseline could be seen at 4 week after an intravitreal injection of 1.25 mg of bevacizumab (B).
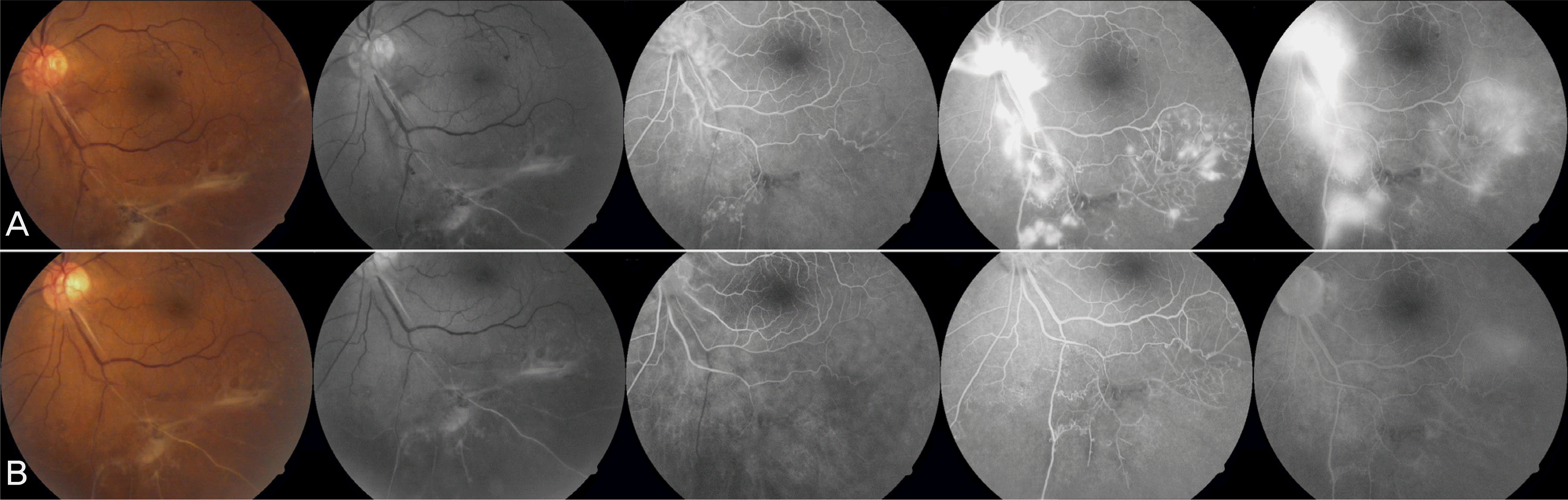
Figure 10.
Color fundus photographs from diabetic patient (case 15) with active new vessels at disc (NVD), dense fibrovascular tissue taken at baseline (A), and at 1 week (B), 4 week (C) after intravitreal bevacizumab injection. Regression of NVD observed at baseline is seen at week 1. But 4 week after intravitreal injection, the development of tractional retinal detachment (TRD) was observed and underwent vitrectomy. After surgery, NVD and dense fibrovascular tissue was completely removed (D).

Table 1.
Clinical charateristics, total area of active new vessel (mm2) and best corrected visual acuity (logMAR§) at baseline and during the 8-week study period
| Case | Sex | Age | DM∗ Type | DM Duration |
Baseline |
Week 1 |
Week 4 |
Week 8 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NV† area | BCVA‡ | NV area | BCVA | NV area | BCVA | NV area | BCVA | |||||
| 1 | M | 59 | II | 19 | 2.21 | 0.52 | 0 | 0.22 | 0 | 0.30 | 0 | 0.52 |
| 2 | M | 63 | II | 11 | 12.30 | 0.40 | 0 | 0.4 | 0 | 0.30 | 3.75 | 0.22 |
| 3 | M | 40 | II | 10 | 13.31 | 1.30 | 0 | 1.10 | 0 | 1.10 | 0.95 | 1.30 |
| 4 | M | 40 | II | 10 | 6.94 | 0.40 | 0 | 0.40 | 0 | 0.30 | 4.89 | 0.30 |
| 5 | M | 49 | II | 12 | 10.57 | 0.30 | 3.01 | 0.30 | 0 | 0.22 | 4.59 | 0.40 |
| 6 | F | 51 | II | 13 | 63.89 | 1.70 | 10.44 | 1.70 | 0 | 1.70 | 16.77 | 1.70 |
| 7 | F | 36 | II | 9 | 34.56 | 0.00 | 8.83 | 0.22 | 0 | 0.00 | 4.41 | 0.04 |
| 8 | F | 63 | II | 16 | 26.01 | 0.30 | 0 | 0.30 | 0 | 0.22 | 7.89 | 0.30 |
| 9 | M | 31 | I | 18 | 9.73 | 0.52 | 0 | 0.52 | 0 | 0.40 | 0 | 0.40 |
| 10 | M | 29 | I | 16 | 6.33 | 0.10 | 0 | 0.04 | 0 | 0.10 | 1.30 | 0.10 |
| 11 | M | 30 | I | 18 | 59.20 | 0.30 | 8.85 | 0.30 | 0 | 0.40 | 13.54 | 0.40 |
| 12 | M | 47 | II | 23 | 81.36 | 1.30 | 40.41 | 1.30 | 0 | 1.30 | 15.13 | 1.30 |
| 13 | F | 52 | II | 12 | 14.14 | 0.10 | 0 | 0.22 | 0 | 0.10 | 6.67 | 0.00 |
| 14 | F | 35 | I | 19 | 10.17 | 1.40 | 2.33 | 1.30 | 0 | 0.52 | 2.08 | 0.52 |
| 15 | M | 61 | II | 15 | 35.63 | 1.10 | 8.99 | 1.15 | 22.21 | 3.00 | 0 | 1.40 |
| 16 | M | 36 | I | 21 | 17.49 | 0.70 | 5.71 | 0.52 | 0 | 0.40 | 4.09 | 0.52 |
| 17 | M | 48 | II | 10 | 21.05 | 0.22 | 3.02 | 0.10 | 0 | 0.10 | 1.04 | 0.10 |
| 18 | F | 70 | II | 45 | 22.66 | 0.15 | 7.67 | 0.15 | 0 | 0.10 | 1.79 | 0.22 |
| 19 | F | 41 | II | 3 | 4.09 | 0.50 | 0 | 0.50 | 0 | 0.00 | 0 | 0.00 |
| 20 | M | 53 | I | 30 | 8.77 | 0.50 | 0 | 0.50 | 0 | 0.50 | 2.05 | 0.50 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download