Abstract
Purpose
To evaluate the long-term surgical results of 120 endoscopic conjunctivodacryocystorhinostomy (CDCR) procedures using a porous polyethylene (MEDPOR®) coated tear drain (MCTD®).
Methods
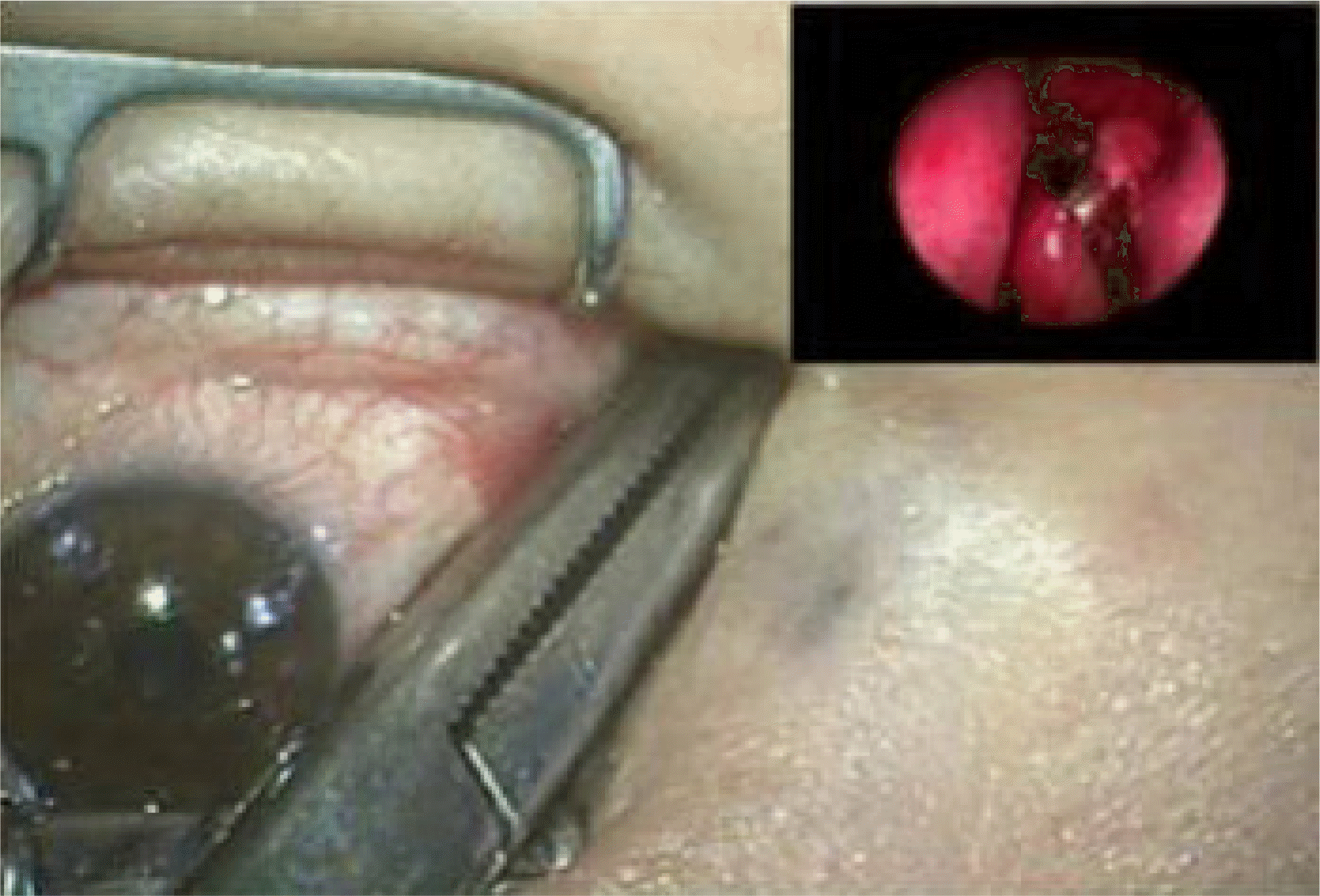
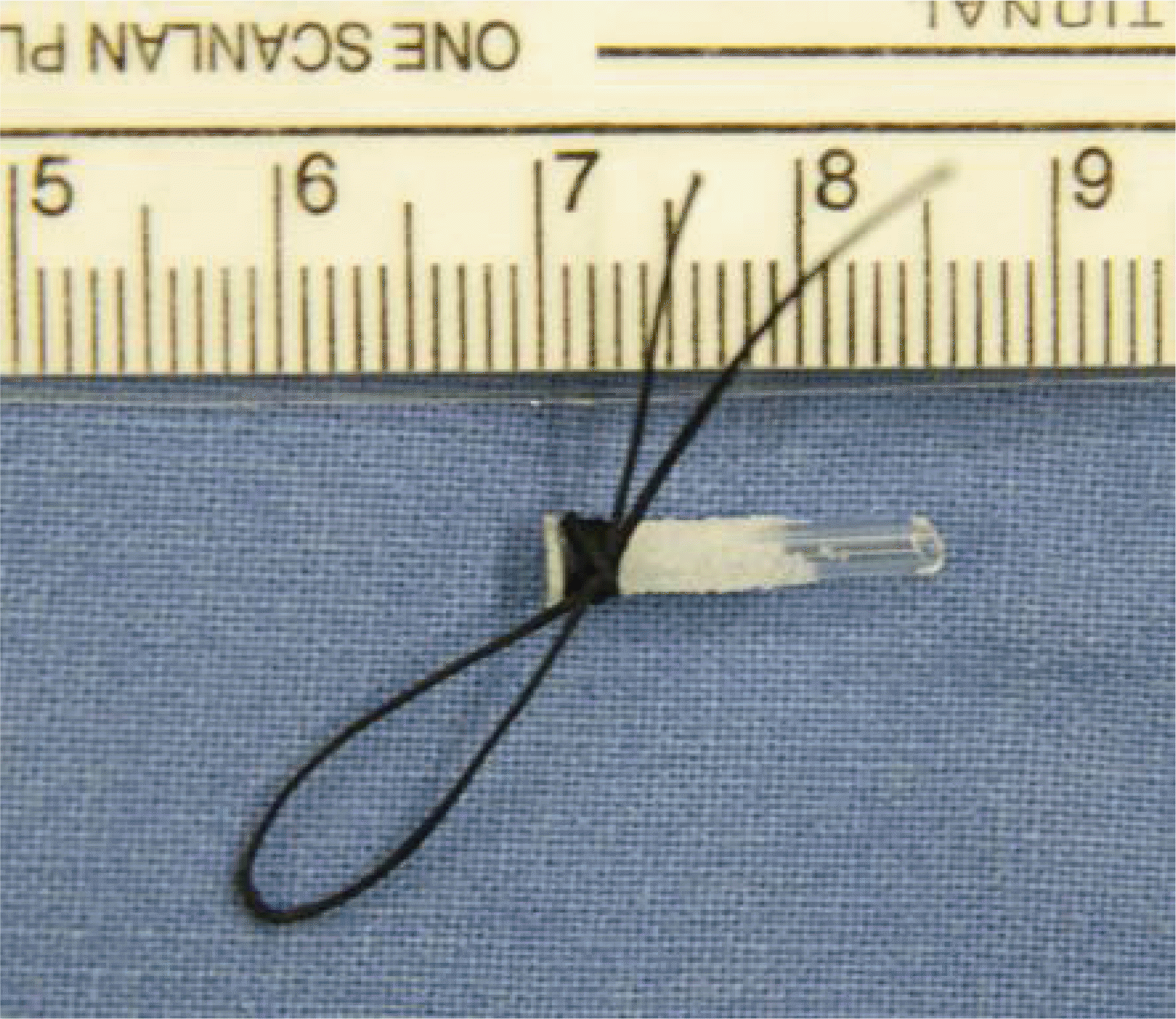
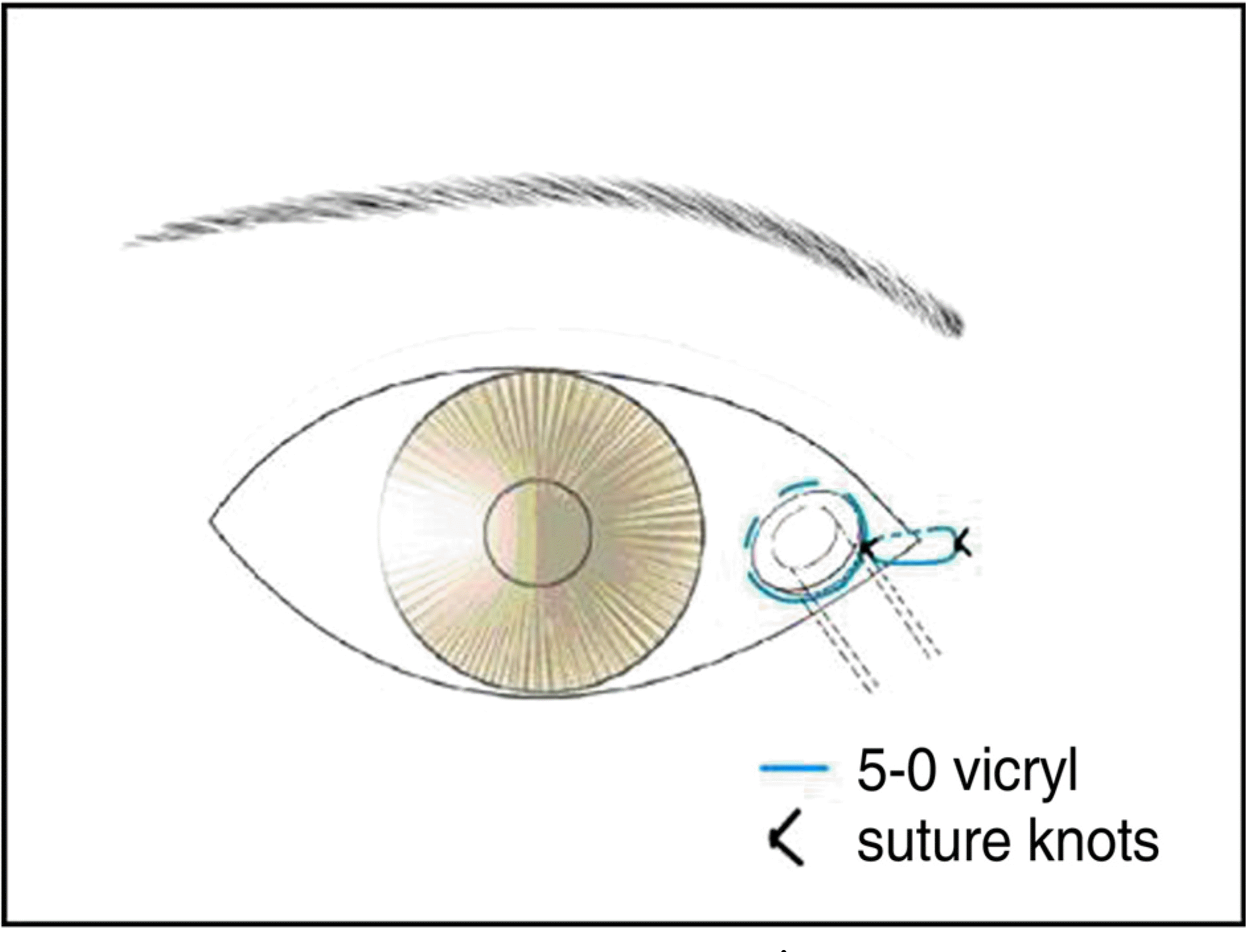
From 2002 to 2007, 120 patients who had been treated with endoscopic CDCR using MCTD® were investigated for its success rates and complications. After an osteotomy was made under nasal endoscopy, a tunnel was created from the caruncle in the conjunctival sac to the nasal cavity through the newly created ostium, and then the tunnel was enlarged to allow the insertion of the MCTD® The length of the tube to be inserted was determined under endoscopic examination. An anchoring suture was placed at the medial canthus to both the conjunctiva and the skin using a 5-0 Vicryl suture in the fashion of a purse string suture.
Results
Causes of obstruction included failed dacryocystorhinostomy (DCR) (74 cases) and idiopathic obstruction (22 cases). Postoperative complications were encountered in four cases with tube loss, 21 cases with a buried tube, 8 cases of extrusion to the conjunctival side, and 11 cases with obstruction caused by conjunctival incarceration or granuloma. The postoperative success rate was 89.1%.
Conclusions
The authors concluded that endoscopic CDCR using MCTD® is an alternative to the standard conventional method for preventing dislodgement of the tube postoperatively. However, surgeons should consider that it may be challenging to insert the MCTD® and that in cases requiring removal, the tube can be difficult to remove due to adhesions.
References
2. Olson JR, Youngs NA. Canaliculusreconstruction with homogenous vein graft. Am J Ophthalmol. 1966; 62:676–7.
3. Reinecke RD, Carroll JM. Silicone lacrimal tube implantation. Trans Am Acad Ophthalmol Otolaryngol. 1969; 73:85–90.
4. Hurwitz JJ. Teflon tubes for stenting and bypassing the lacrimal drainage pathways. Ophthalmic Surg. 1989; 20:855–9.

5. Sekhar GC, Dortzbach RK, Gonnering RS, Lemke BN. Problems associated with conjunctivodacryocystorhinostomy. Am J Ophthalmol. 1991; 112:502–6.

6. Steinsapir KD, Glatt HJ, Putterman AM. A 16-year study of conjunctival dacryocystorhinostomy. Am J Ophthalmol. 1990; 109:387–93.

7. Lee TS, Oh IK, Kim JS. The Clinical Outcome of Endoscopic Transnasal Conjunctivodacryocystorhinostomy Using MEDPOR® Coated Tear Drain. J Korean Ophthalmol Soc. 2004; 45:1420–6.
8. Lee TS, Lee H. Purse-string Suture Technique for Jones Tube Fixation in conjunctivodacryocystorhinostomy. J Korean Ophthalmol Soc. 2008; 49:1553–8.

9. Rose GE, Welham RA. Jones’ lacrimal canalicular bypass tubes: Twenty-five years’ experience. Eye. 1991; 5:13–9.

10. Olver J. Color atlas of lacrimal surgery. 1st ed.Oxford: Butterworth- Heinermann;2001. p. 162–74.
11. Glastone GJ, Putterman AM. A modified glass tube for conjunctivodacryocystorhinostomy. Arch Ophthalmol. 1985; 103:1229–30.
12. Corin SM, Hurwitz JJ, Tucker SM. A simple technique for the prevention and management of Jones bypass tube extrusion. Can J Ophthalmol. 1988; 23:322–3.
13. Henderson PN. A modified trephining technique for the insertion of Jones tube. Arch Ophthalmol. 1985; 103:1582–5.

14. Colla B, Riestra J, Missotten L. A modified Jones tube. Bull Soc Belge Ophtalmol. 1996; 261:53–6.
16. Kim YS, Lee TS. Clinical Study Conjunctivodacryocystorhinostomy with Jones Tube. J Korean Ophthalmol Soc. 1991; 32:129–33.
17. Yun JR, Chang HK. Long-ternm followup of Conjunctivodacryocystorhinostomy. J Korean Ophthalmol Soc. 1996; 37:1583–9.
18. Lim C, Martin P, Benger R, et al. Lacrimal canalicular bypass surgery with the Lester Jones tube. Am J Ophthalmol. 2004; 137:101–8.

19. Chung HS, Han DK, Lee KY. Conjunctivodacryocystorhinostomy with Jones Tube. J Korean Ophthalmol Soc. 1985; 26:215–9.
Table 1.
Size of tube used (mm)
| Length of tube (mm) | Number of cases |
|---|---|
| 16 | 9 |
| 17 | 14 |
| 18 | 20 |
| 19 | 17 |
| 20 | 14 |
| 22 | 10 |
| 24 | 4 |
| Total | 120 |
Table 2.
Causes of CDCR∗ in 120 eyes
| Causes | Number of patients (%) |
|---|---|
| Failed DCR† | 74 (61.6) |
| Idiopathic | 22 (18.3) |
| Trauma | 16 (13.3) |
| Congenital | 3 (2.5) |
| Tumor | 3 (2.5) |
| Inflammation | 2 (1.5) |
| Total | 120 (100) |
Table 3.
Success rates of endoscopic CDCR∗ using MCTD®
| Number of cases | % | |
|---|---|---|
| Excellent | 86 | 71.6 |
| Good | 21 | 17.5 |
| Poor | 13 | 10.9 |
Table 4.
Postoperative complications of endoscopic CDCR∗ using MCTD®
| Complications | Numberof cases (%) |
|---|---|
| Displacement of tube | 33 (27.5) |
| Buried tube | 21 (17.5) |
| Extrusion of tube | 8 (6.7) |
| Loss of tube | 4 (3.3) |
| Obstruction of tube | 11 (9) |
| Infection | 3 (2.5) |
| Broken tube | 1 (0.8) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download