Abstract
Purpose
To investigate the cellular protective effects of hypoxic preconditioning against oxidative stress in a staurosporine-differentiated RGC-5 cell line and the relevance of protein kinase C subtype expression.
Methods
The minimum staurosporine concentration and exposure time necessary to morphologically fully differentiate RGC-5 cells were determined. Cytotoxic injury was provided by oxidative stress with 800 µM hydrogen peroxide (H2 O2) for 15 hours to morphologically fully-differentiated cells. The cytoprotective effect of hypoxic preconditioning was found by exposing the cell line to 0.3% oxygen for different periods of time. Quantifiable changes in the expression of mRNAs and proteins of the isoenzymes α, β, γ, δ, ε, ζ of protein kinase C were determined before and after 1, 2, 15, and 24 hours of hypoxic preconditioning.
Results
Axonal growth in RGC-5 cells after the induction of differentiation with staurosporine caused these cells to resemble neurons. The minimal concentration and exposure time to staurosporine that evoked full differentiation of RGC-5 cells was exposure to 2 µM staurosporine for 1 hour. An LDH assay demonstrated that hypoxic preconditioning had neuroprotective effects against hydrogen peroxide-induced oxidative stress. Protein and mRNA levels of PKC isoforms α and ε increased after preconditioning.
Conclusions
Hypoxic preconditioning of staurosporine-differentiated RGC-5 cells had a cytoprotective effect against oxidative stress. The associated increase of mRNA and proteins of PKC isoenzymes α and ε suggest some functional relevance of these isoenzymes to the cytoprotective effects conferred by hypoxic preconditioning.
References
1. Goldberg I. Weinreb RN, Kitazawa J, Krieglestein GK, editors. How common is glaucoma worldwide? Glaucoma in the 21st Century. Landau, Germany: Mosby;2000:1–8.
2. Giammanco R, Dardanoni G, Ponte F.Prevalence of glaucoma in a population. Acta Ophthalmol Scand. 1995; 73:222–5.
3. Cedrone C, Culasso F, Cesareo M. . Prevalence of glaucoma in Ponza. Ophthalmic Epidemiol. 1997; 4:59–72.
4. Owsley C, McGwin G Jr, Ball K. Vision impairment, eye disease, and motor vehicle crashes in the elderly. Ophthalmic Epidemiol. 1998; 5:101–13.
5. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130:429–40.
6. Collaborative Normal-Tension Glaucoma Study Group (CNTGSP). Comparison of glaucomatous progression between untreated patients with normal‐ tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998; 126:487–97.
8. Flammer J, Haefliger IO. Orgül S, Resink T. Vascular ysregulation: a principal risk factor for glaucoma damage? J Glaucoma. 1999; 8:212–9.
9. Dowden J, Corbett D. Ischemic preconditioning in 18 to 20 month‐ old gerbils long‐ term survival with functional outcome measures. Stroke. 1999; 30:1240–6.
10. Kristian T. Siesjö BK. Calcium in ischemic cell death. Stroke. 1998; 29:705–18.
12. Hawaleshka A, Jacobsohn E. Ischemic preconditioning: mechanisms and potential clinical applications (Review). Can J Anaesth. 1998; 45:670–82.
13. Kitagawa K, Matsumoto M, Tagaya M. . Ischemic tolerance phenomenon found in the brain. Brain Res. 1990; 528:21–4.

14. Paratt JR. Protection of the heart by ischemic preconditioning: mechanisms and possibilities for pharmacological expression. Trends Pharmacol Sci. 1994; 15:19–25.
15. Barone FC, White RF, Spera PA. . Ischemic preconditioning and brain tolerance. Stroke. 1998; 29:1937–51.
16. Zhu Y, Ohlemiller KK, McMahan BK. . Constitutive nitric oxide synthase activity is required to trigger ischemic tolerance in mouse retina. Exp Eye Res. 2006; 82:153–63.

17. Ozbay D, Ozden S, Muftuoglu S. . Protective effect of ischemic preconditioning on retinal ischemia‐ reperfusion injury in rats. Can J Ophthalmol. 2004; 39:727–32.
18. Sakamoto K, Yonoki Y, Kuwagata M. . Histological protection against ischemia‐ reperfusion injury by early ischemic preconditioning in rat retina. Brain Res. 2004; 1015:154–60.
19. Matsumoto S, Shamloo M, Matsumoto E. . Protein kinase C-gamma and calcium/calmodulin-dependent protein kinase II alpha are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2004; 24:54–61.
20. Speechly‐ Dick ME, Mocanu MM, Yellon DM, Protein kinase C.Its role in ischemic preconditioning in the rat. Circ Res. 1994; 75:586–90.
21. Piccoletti R, Bendinelli P, Arienti D. Bernelli-Zazzera A. State and activity of protein kinase C in postischemic reperfused liver. Exp Mol Pathol. 1992; 56:219–28.
22. Padanilam BJ. Induction and subcellular localization of protein kinase C isozymes following renal ischemia. Kidney Int. 2001; 59:1789–97.

23. Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994; 17:551–67.

24. Chen L, Hahn H, Wu G. . Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001; 98:11114–9.
25. Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF‐1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005; 46:1092–8.

26. Charles I, Khalyfa A, Kumar DM. . Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005; 46:1330–8.

27. Maher P, Hanneken A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2005; 46:749–57.

28. Krishnamoorthy RR, Agarwal P, Prasanna G. . Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001; 86:1–12.
Figure 1.
The assessment of optimal level and duration in staurosporine treatment for differentiation of RGC-5: The shape of RGC-5 cell changes from fibroblast-like shape to neuron-like shape showing axon and multiple synapse with treatment of staurosporine. Staurosporine 1.0 µg for 2 hours seemed to be the minimum duration and the level for the appropriate morphological differentiation. Staurosporine 0 µg: 1 hr (A), 2 hr (B), 6 hr (C), 24 hr (D); Staurosporine 0.5 µg: 1 hr (E), 2 hr (F), 6 hr (G), 24 hr (H); Staurosporine 1.0 µg: 1 hr (I), 2 hr (J), 6 hr (K), 24 hr (L); Staurosporine 2.0 µg: 1 hr (M), 2 hr (N), 6 hr (O), 24 hr (P).

Figure 2.
Cytotoxicity of ischemic preconditioning (0.3% oxygen state) against oxidative stress (800 µM H2 O2 for 15 hr) in differentiated RGC-5 as demonstrated with LDH assay. With prolonged duration of ischemic preconditioning, RGC-5 survival increased up to 8 hrs of ischemic preconditioning after which cytotoxicity increased. Cytotoxicity after oxidative stress was statistically significantly decreased at 2 to 24 hours of ischemic preconditioning when compared to control (* p<0.05 by Mann-Whitney U test).
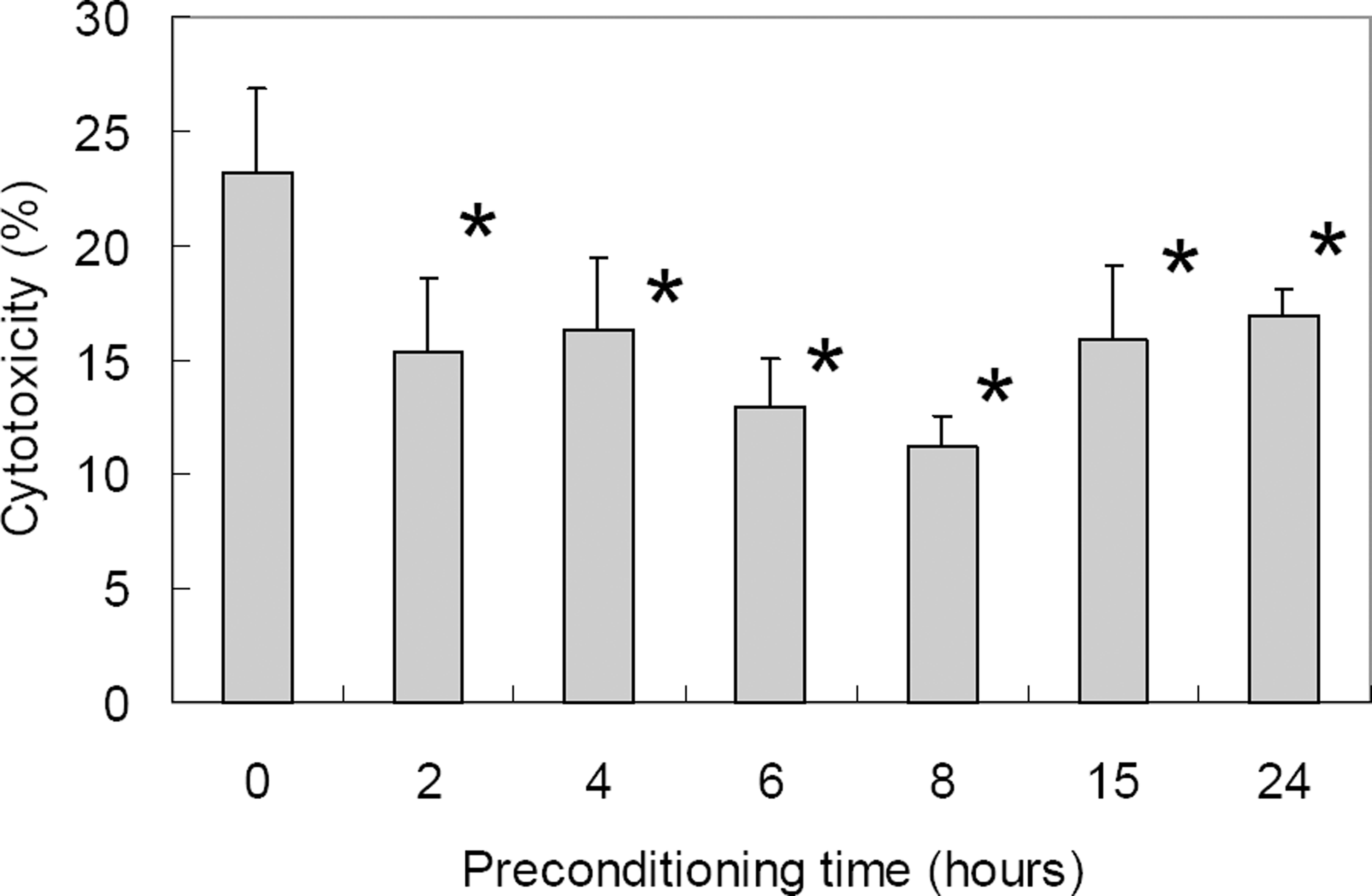
Figure 3.
Changes of PKC iso-enzyme mRNA before and after ischemic preconditioning (B-PC, before preconditioning; PC0hr, just after the preconditioning; PC1hr, 1 hour after preconditioning; PC2hr, 2 hours after preconditioning; PC15hr, 15 hours after preconditioning; PC24hr, 24 hours after preconditioning); We can see the elevation of level of PKC α, β, γ, δ, ε mRNA 1 or 2 hours after PC (preconditioning, 0.3% oxygen 8 hrs). The elevations are decreasing with time after PC.
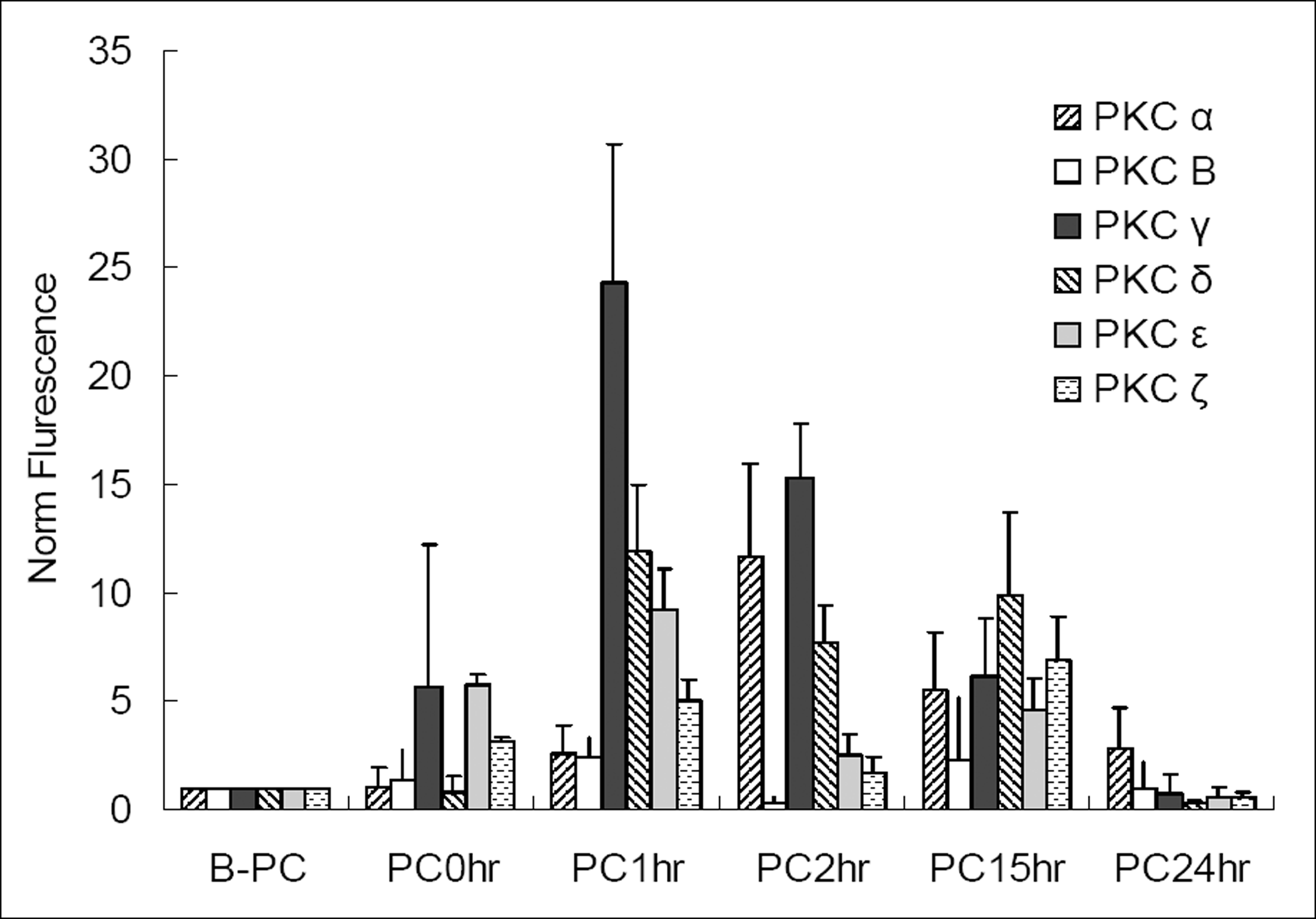
Figure 4.
Changes of PKC iso-enzyme protein before and after ischemic preconditioning (B, before preconditioning; 0, just after the preconditioning; 1, 1 hour after preconditioning; 2, 2 hours after preconditioning; 15, 15 hours after preconditioning; 24, 24 hours after preconditioning). PKC ε increased until 2 hours after the preconditioning, and PKC α increased from 15 hours after the preconditioning. These changes are relatively correlated with increasing tendency of mRNA.
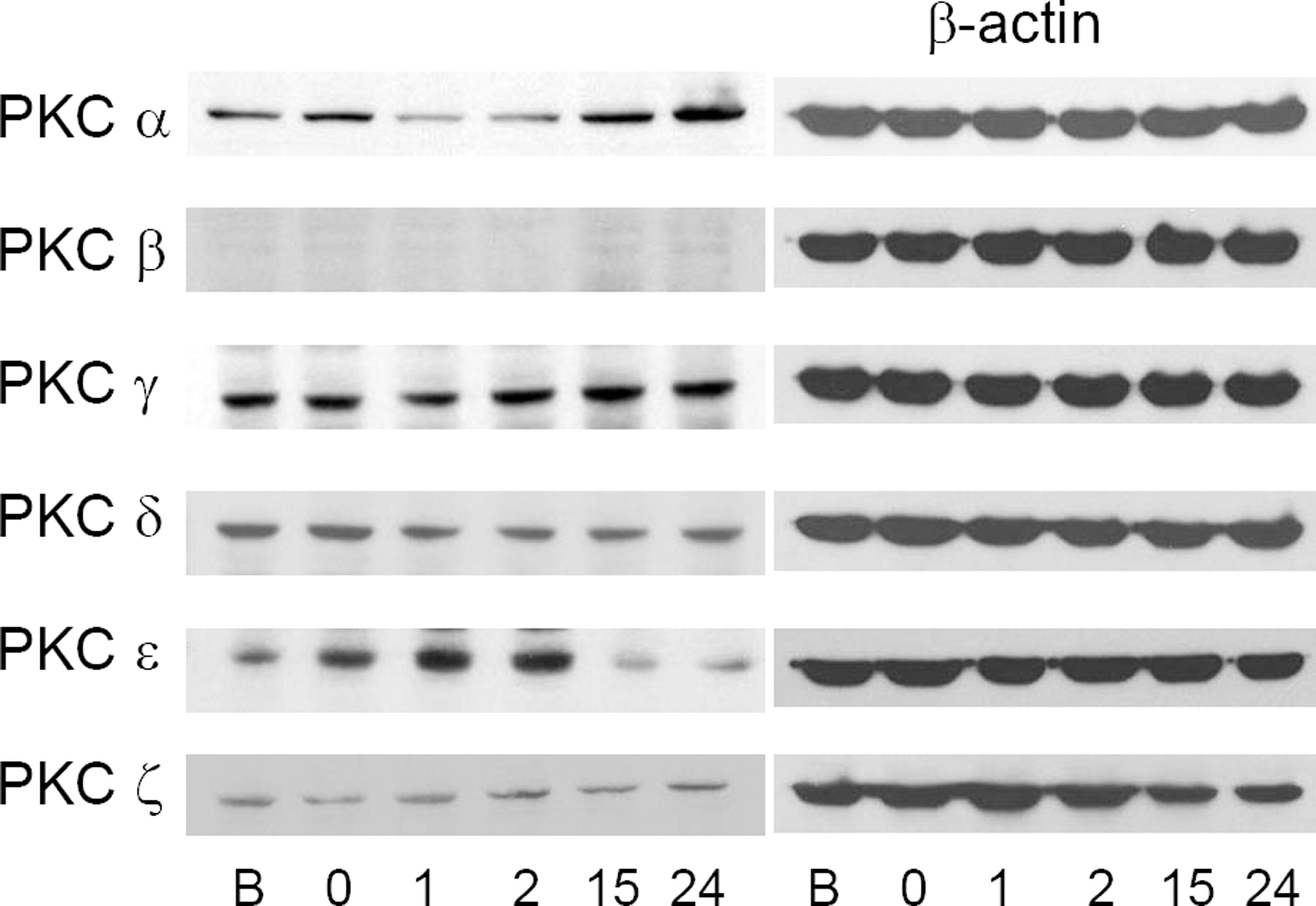
Table 1.
Primer sequences for real-time PCR (F=forward; R=reverse)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download